Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters - PubMed (original) (raw)
Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters
Akiko Tagawa et al. J Cell Biol. 2005.
Abstract
Using total internal reflection fluorescence microscopy (TIR-FM), fluorescence recovery after photobleaching (FRAP), and other light microscopy techniques, we analyzed the dynamics, the activation, and the assembly of caveolae labeled with fluorescently tagged caveolin-1 (Cav1). We found that when activated by simian virus 40 (SV40), a non-enveloped DNA virus that uses caveolae for cell entry, the fraction of mobile caveolae was dramatically enhanced both in the plasma membrane (PM) and in the caveosome, an intracellular organelle that functions as an intermediate station in caveolar endocytosis. Activation also resulted in increased microtubule (MT)-dependent, long-range movement of caveolar vesicles. We generated heterokaryons that contained GFP- and RFP-tagged caveolae by fusing cells expressing Cav1-GFP and -RFP, respectively, and showed that even when activated, individual caveolar domains underwent little exchange of Cav1. Only when the cells were subjected to transient cholesterol depletion, did the caveolae domain exchange Cav1. Thus, in contrast to clathrin-, or other types of coated transport vesicles, caveolae constitute stable, cholesterol-dependent membrane domains that can serve as fixed containers through vesicle traffic. Finally, we identified the Golgi complex as the site where newly assembled caveolar domains appeared first.
Figures
Figure 1.
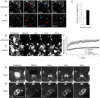
Mobility of surface and caveosomal Cav1-GFP can be activated. (A and B) Dynamics of surface Cav1-GFP recorded by TIR-FM in untreated CV-1 cells expressing Cav1-GFP (A), or 1 h after addition of SV40 (MOI 103; B). The images shown are snapshots from Video 1 (available at
http://www.jcb.org/cgi/content/full/jcb.200506103/DC1
). The video was recorded for 2 min at 4 hertz. Red and blue arrowheads indicate appearing and disappearing vesicles, respectively. Note that an increased number of docking and leaving vesicles are observed in the presence of SV40 (see Video 1). Bars, 1 μm. (C) The fraction of mobile Cav1 vesicles at the surface recorded by TIR-FM doubles upon addition of SV40. Experiments were performed as described in A and B, and the fraction of Cav1-GFP vesicles that underwent docking or lateral movement at the cell surface was determined as described in Fig. S1. The mean of three independent experimental sets is shown. Error bars indicate standard deviations of the three experiments. (D and E) Individual caveosomes were bleached in CV-1 cells expressing Cav1-GFP. The cells were (D) untreated or (E) bleaching was performed 1 h after addition of SV40 (MOI 60). From left to right, before (Prebleach), immediately after (Bleach), and 1, 2, and 10 min after bleaching are shown. The circles show the bleached areas. Note that little recovery occurs in the absence of SV40 (see Video 2). Bars, 2 μm. (F) FRAP curves for individual caveosomes in CV-1 cells expressing Cav1-GFP. The cells were either untreated, exposed to SV40 (MOI 60) for 1 h, to 100 μM genistein for 30 min and then 1 h to SV40 (MOI 60), or to 1 mM vanadate for 1 h before the FRAP experiments. Fluorescence recovery was recorded every 10 s for 10 min. Error bars indicate standard deviations of five experiments for untreated, +SV40, vanadate, and three for +SV40/+genistein. Note that in the presence of SV40 or vanadate, the recovery rate is high, and that preincubation with genistein brings the recovery rate back to the slow rate seen in untreated cells. (G) Cav1-GFP does not diffuse laterally in caveosomes. A part of a caveosome (dashed rectangle) was bleached in a CV-1 cell expressing Cav1-GFP. Recovery of fluorescence was recorded every 6 s for 6 min. Before (Prebleach), immediately after (Bleach), and 2, 4, and 6 min after bleaching are shown. Note the slow and incomplete fluorescence recovery, and the lack of lateral redistribution of Cav1-GFP–labeled domains (see Video 3). Bar, 2 μm. (H) A small portion of an endosome (dashed rectangle) was bleached in a CV-1 cell expressing Rab 7-GFP and recovery was recorded as in G. Note that despite the small area bleached, fluorescence in the entire endosome was eliminated, and that recovery was rapid and complete (see Video 4). Bar, 2 μm.
Figure 2.
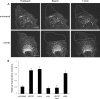
Cav1-containing structures move long distances in activated cells. (A) Large peripheral areas of CV-1 cells expressing Cav1-GFP were bleached (marked areas in bleach panels), and the movement of Cav1-GFP into the bleached area was monitored omitting the 5-μm region closest to the bleach boundary (marked in 15 min panels). The experiment was performed in the absence (untreated, upper panels) and the presence of SV40 (1 h incubation, MOI 60; +SV40, lower panels). Before (Prebleach), immediately after (Bleach), and 15 min after (15 min) bleaching are shown. Note the increase in long-distance movement in the presence of SV40 (see Videos 5 and 6, available at
http://www.jcb.org/cgi/content/full/jcb.200506103/DC1
). Bars, 10 μm. (B) Recovery of fluorescence due to the long-distance movement of Cav1-GFP in CV-1 cells increases after addition of SV40, vanadate, or latA. The CV-1 cells expressing Cav1-GFP were either untreated, exposed to SV40 (MOI 60) for 1 h, to 1 mM vanadate for 1 h, to 5 μM nocodazole for 30 min, to 5 μM nocodazole for 30 min and then 1 h to SV40 (MOI 60), or to 0.8 μM latA for 10 min before the FRAP experiments. The fluorescence recovery was quantified in the bleached area omitting the 5 μm region closest to the bleach boundary (marked in 15 min panels in A) after 15 min. Recovery was calculated by measuring the fluorescence intensity in the defined area before and 15 min after bleaching (see Videos 5–7). The error bars indicate standard deviations of five independent experiments.
Figure 3.
Cav1-GFP and -RFP become mobile in stimulated heterokaryons. (A–H) HeLa cells expressing Cav1-GFP (A) were fused with the ones expressing Cav1-RFP (B), or the cells expressing clathrin light chain–GFP (E) were fused with cells expressing clathrin light chain–RFP (F) by 2 min PEG treatment (merge, C and G, respectively). The cells were incubated in the presence of CHX after fusion, fixed after 3 h, and viewed by confocal microscopy. The dashed circles show the positions of the nuclei. Note that in C, the Cav1 spots remain either red or green, and that only few spots have moved across the fusion boundary. In G, even distribution and colocalization of the green and red clathrin is apparent throughout the cell. (D and H) The mixing of Cav1-GFP and -RFP in heterokaryons was enhanced upon stimulation. HeLa cells expressing Cav1-GFP and -RFP were fused and exposed to stimuli. SV40 (MOI 103; D) or 1 mM vanadate (H) was added at 1.5 h after PEG-induced fusion, and cells were incubated for another 1.5 h before fixation. Note extensive mixing of Cav1-GFP and -RFP throughout the cell. Bar, 10 μm. (I) Quantification of caveolar mobility from the experiments in C, D, G, and H. See Fig. S2 for details.
Figure 4.
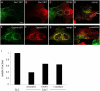
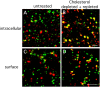
Cav1 does not exchange between caveolar domains. (A) Dual-color TIR-FM images at the cell surface of HeLa heterokaryons expressing Cav1-GFP and -RFP, in the presence of stimuli. SV40 (MOI 103, left) or vanadate (1 mM, right) were added 1.5 h after fusion, and cells were incubated for another 1.5 h before fixation. Bars, 2 μm. (B–E) Distribution of Cav1--GFP and -RFP (B–D) or clathrin light chain–GFP and –RFP (E) was imaged on confocal microscope 3 h after fusion. In the absence (B) and presence of SV40 (C; MOI 103, added at 1.5 h), Cav1-GFP and -RFP coexpressed (no fusion, D), or clathrin light chain–GFP and –RFP at 3 h after fusion (E). Note the individual red and green spots in the absence or presence of SV40 (B and C; and Video 8, available at
http://www.jcb.org/cgi/content/full/jcb.200506103/DC1
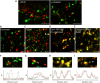
), and the yellow spots indicating complete colocalization after coexpression of Cav1-GFP and -RFP (D), and in fusion of clathrin light chain–GFP and –RFP (E). Note a caveosome with a mosaic of red, green, and yellow regions in C (arrowhead), whereas in B, there are either red or green caveosomes and very few caveosomes containing both colors. Bars, 2 μm. (F–I) Zoomed-in images of caveosomes (upper panels) prepared as in B–E above, and corresponding fluorescence intensity curves of GFP and RFP (graphs below). In the absence (F) and presence of SV40 (G; and Video 8), Cav1-GFP and -RFP coexpressed (no fusion, H) or clathrin light chain–GFP and –RFP at 3 h after fusion (I). Fluorescence intensity of GFP and RFP along the line across the caveosome structures were measured (RI, relative fluorescence intensity) and directly plotted against the distance (red line, RI of RFP; green line, RI of GFP).
Figure 5.
Cholesterol depletion and repletion allow mixing of Cav1-GFP and -RFP. (A–D) Distribution of Cav1-GFP and -RFP in heterokaryons of HeLa cells expressing Cav1-GFP and -RFP, respectively. (A) An intracellular view of a heterokaryon in untreated control imaged by confocal microscopy. Fused cells were incubated in the presence of CHX, and were imaged 5 h after fusion. (B) An intracellular view of a heterokaryon after cholesterol depletion and repletion imaged by confocal microscopy. The cells were fused, recovered for 1 h, treated with cholesterol-depleting drugs nystatin (25 μg/ml) and progesterone (10 μg/ml) for 2 h, repleted with cholesterol by addition of 10% FCS for 2 h, and imaged 5 h after fusion. Bar, 2 μm. (C) A surface view of untreated heterokaryon (same condition as in A) was imaged by TIR-FM 5 h after fusion. (D) A surface view of a heterokaryon after cholesterol depletion and repletion (same condition as in B) was imaged by TIR-FM 5 h after fusion. Note in B, there are caveosomes that reassembled into completely yellow caveosomes, and in D, yellow surface caveolae that presumably transported from intracellular pool to surface are visible. Bar, 2 μm.
Figure 6.
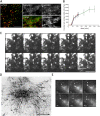
Newly assembled caveolar domains in the Golgi complex and in transit to the PM. (A) Appearance of yellow Cav1 in the heterokaryons of HeLa cells expressing Cav1-GFP and -RFP, respectively, was detected on the PM (surface) by TIR-FM as well as in the Golgi complex by confocal microscopy in the absence of CHX, 3 h after fusion. Cav1-GFP, -RFP, merged image of Cav1-GFP and -RFP (merge), and anti-Giantin are shown. Bars, 5 μm. (B) Kinetics of Cav1 appearance on the PM in CV-1 cells expressing Cav1-GFP observed by TIR-FM. The number of spots in three homogenously illuminated areas per cell was counted, normalized to the average total visible cell surface (671 ± 228 μm2, disregarding indentations and non-flat areas of the PM), and plotted against time. Red, blue, and green lines represent three independent experiments, and the error bars are SDs at each time point within each set of experiment. (C) Spinning disc confocal images of Cav1 structures leaving from the Golgi complex in CV-1 cells expressing Cav1-GFP (arrowheads). Images taken at 2 Hz (0–4 s) (Video 9, available at
http://www.jcb.org/cgi/content/full/jcb.200506103/DC1
) and a projected image over the 4 s (projection) are shown. Bar, 5 μm. (D) Cav1 structures leaving from the Golgi complex to the PM in CV-1 cells expressing Cav1-GFP were imaged at 1 Hz for 300 frames on the TIR-FM, but with illumination such that cell surface and part of the Golgi complex were visible simultaneously (Video 10). Consecutive frames were subtracted from each other to yield a stack of images with only moving objects. The frames were projected, and trajectories generated this way are shown. Note that the Cav1 spots in the trajectories do not change their appearance (arrowheads). Bar, 10 μm. (E) Cav1 structures leaving from the Golgi complex area (cloudy staining in the background) and arriving on the cell surface (arrowheads) in CV-1 cells expressing Cav1-GFP. Images were taken as in D (Video 10), and selected frames (0, 3, 7, 9, 19, and 46 s) are shown. Bar, 2 μm.
Similar articles
- Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic.
Pelkmans L, Bürli T, Zerial M, Helenius A. Pelkmans L, et al. Cell. 2004 Sep 17;118(6):767-80. doi: 10.1016/j.cell.2004.09.003. Cell. 2004. PMID: 15369675 - Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER.
Pelkmans L, Kartenbeck J, Helenius A. Pelkmans L, et al. Nat Cell Biol. 2001 May;3(5):473-83. doi: 10.1038/35074539. Nat Cell Biol. 2001. PMID: 11331875 - Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface.
Choudhury A, Marks DL, Proctor KM, Gould GW, Pagano RE. Choudhury A, et al. Nat Cell Biol. 2006 Apr;8(4):317-28. doi: 10.1038/ncb1380. Epub 2006 Mar 26. Nat Cell Biol. 2006. PMID: 16565709 - Caveolae/raft-dependent endocytosis.
Nabi IR, Le PU. Nabi IR, et al. J Cell Biol. 2003 May 26;161(4):673-7. doi: 10.1083/jcb.200302028. J Cell Biol. 2003. PMID: 12771123 Free PMC article. Review. - Caveolins and caveolae: molecular and functional relationships.
Razani B, Lisanti MP. Razani B, et al. Exp Cell Res. 2001 Nov 15;271(1):36-44. doi: 10.1006/excr.2001.5372. Exp Cell Res. 2001. PMID: 11697880 Review. No abstract available.
Cited by
- Dynamic architecture of the purinosome involved in human de novo purine biosynthesis.
Kyoung M, Russell SJ, Kohnhorst CL, Esemoto NN, An S. Kyoung M, et al. Biochemistry. 2015 Jan 27;54(3):870-80. doi: 10.1021/bi501480d. Epub 2015 Jan 15. Biochemistry. 2015. PMID: 25540829 Free PMC article. - Caveolin targeting to late endosome/lysosomal membranes is induced by perturbations of lysosomal pH and cholesterol content.
Mundy DI, Li WP, Luby-Phelps K, Anderson RG. Mundy DI, et al. Mol Biol Cell. 2012 Mar;23(5):864-80. doi: 10.1091/mbc.E11-07-0598. Epub 2012 Jan 11. Mol Biol Cell. 2012. PMID: 22238363 Free PMC article. - Internalization of the TGF-β type I receptor into caveolin-1 and EEA1 double-positive early endosomes.
He K, Yan X, Li N, Dang S, Xu L, Zhao B, Li Z, Lv Z, Fang X, Zhang Y, Chen YG. He K, et al. Cell Res. 2015 Jun;25(6):738-52. doi: 10.1038/cr.2015.60. Epub 2015 May 22. Cell Res. 2015. PMID: 25998683 Free PMC article. - Caveolin-1 is an aggresome-inducing protein.
Tiwari A, Copeland CA, Han B, Hanson CA, Raghunathan K, Kenworthy AK. Tiwari A, et al. Sci Rep. 2016 Dec 8;6:38681. doi: 10.1038/srep38681. Sci Rep. 2016. PMID: 27929047 Free PMC article. - Model for the architecture of caveolae based on a flexible, net-like assembly of Cavin1 and Caveolin discs.
Stoeber M, Schellenberger P, Siebert CA, Leyrat C, Helenius A, Grünewald K. Stoeber M, et al. Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):E8069-E8078. doi: 10.1073/pnas.1616838113. Epub 2016 Nov 10. Proc Natl Acad Sci U S A. 2016. PMID: 27834731 Free PMC article.
References
- Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe, N. Itoh, M. Shibuya, and Y. Fukami. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592–5595. - PubMed
- Bonifacino, J.S., and B.S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell. 116:153–166. - PubMed
- Brown, D.J., and J.A. Gordon. 1984. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J. Biol. Chem. 259:9580–9586. - PubMed
- Conner, S.D., and S.L. Schmid. 2003. Regulated portals of entry into the cell. Nature. 422:37–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous