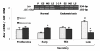Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes - PubMed (original) (raw)
Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes
M Cecilia Johnson et al. Reprod Biol Endocrinol. 2005.
Abstract
Background: Endometriosis is a common gynaecological disorder characterized by the presence of endometrial tissue outside of the uterus. The fragments in normal menstruation are composed of necrotic and living cells, which do not survive in ectopic locations because of programmed cell death. The aim of this study was to evaluate if the balance between cell proliferation and apoptosis is changed in eutopic endometrium from women with endometriosis throughout the menstrual cycle by studying bax (pro-apoptotic), c-myc (regulator of cell cycle) and TGF-beta1 (involved in cell differentiation) genes.
Methods: Eutopic endometrium was obtained from: 30 women with endometriosis (32.8 +/- 5 years) and 34 fertile eumenorrheic women (36 +/- 5.3 years). We analyzed apoptosis (TUNEL: DNA fragmentation); cell proliferation (immunohistochemistry (IHC) for Ki67); c-myc, bax and TGF-beta1 mRNA abundance (RT-PCR) and TGF-beta1 protein (IHC) in endometrial explants.
Results: Cell proliferation strongly decreased from proliferative to late secretory phases in glands, but not in stroma, in both endometria. Positive staining in glands and stroma from proliferative endometrium with endometriosis was 1.9- and 2.2-fold higher than control endometrium, respectively (p < 0.05). Abundance of c-myc mRNA was 65% higher in proliferative endometrium from endometriosis than normal tissue (p < 0.05). TGF-beta1 (mRNA and protein) augmented during mid secretory phase in normal endometrium, effect not observed in endometrium with endometriosis. In normal endometrium, the percentage of apoptotic epithelial and stromal cells increased more than 30-fold during late secretory phase. In contrast, in endometrium from endometriosis, not only this increase was not observed, besides bax mRNA decreased 63% versus normal endometrium (p < 0.05). At once, in early secretory phase, apoptotic stromal cells increased 10-fold with a concomitant augment of bax mRNA abundance (42%) in endometria from endometriosis (p < 0.05).
Conclusion: An altered expression of c-myc, TGF-beta1 and bax was observed in eutopic endometrium from endometriosis, suggesting its participation in the regulation of cell survival in this disease. The augmented cell viability in eutopic endometrium from these patients as a consequence of a reduction in cell death by apoptosis, and also an increase in cell proliferation indicates that this condition may facilitate the invasive feature of the endometrium.
Figures
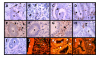
Figure 1
Immunohistochemical staining for Ki67, TGF-β1 and apoptosis by TUNEL in explants of human endometrium throughout the menstrual cycle obtained from normal (A, C, E, G, I, J and K) and endometriosis (B, D, F, H and L) women. Representative human endometrium explants from (A, B, E, F and I) proliferative phase and (C, D, G, H, J, K and L) secretory phase of the menstrual cycle with positive immuno-staining for (A-D) Ki67 and (E-H) TGF-β1 or positive DNA fragmentation by TUNEL (K and L). Immunohistochemitry (I) and TUNEL (J) negative controls. Cell nuclei are stained with haematoxylin (immunohistochemitry) or propidium iodine (TUNEL). g: glandular, s: stroma, rc: red blood cell. Magnification, 400×.
Figure 2
PCR amplification from endometrium cDNA of women without (Normal) and with endometriosis using primers for c-myc (330-bp) and 18S rRNA (192-bp). Representative gel is shown. Graph illustrates the corresponding amplification relative to 18S rRNA and the results are given as mean ± SEM from: 6 and 6 proliferative (P); 6 and 5 early secretory (ES); 5 and 4 mid secretory (MS) and 5 and 4 late secretory (LS) eutopic endometria obtained from women without and with endometriosis, respectively. (-) PCR amplification without template. *p < 0.05 vs. normal endometria.
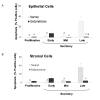
Figure 3
Percentage of apoptotic cells in epithelial (A) and stromal cell (B) compartments in human endometrium explants obtained from normal women and women with endometriosis. Histological sections were evaluated by TUNEL technique (see text). The results are given as mean ± SEM from: 5 and 6 proliferative; 5 and 6 early secretory; 4 and 5 mid secretory; 7 and 6 late secretory eutopic endometria obtained from women without (normal) and with endometriosis, respectively. *p < 0.05 vs. normal endometria. °p < 0.05 vs. early secretory phase. #p < 0.05 vs. late secretory phase.
Figure 4
PCR amplification from endometrium cDNA of women without (Normal) and with endometriosis using primers for bax (334-bp) and 18S rRNA (192-bp). Representative gel is shown. Graph illustrates the corresponding amplification relative to 18S rRNA (192-bp) and the results are given as mean ± SEM from 5 and 5 proliferative (P); 6 and 5 early secretory (ES); 5 and 6 mid secretory (MS) and 4 late secretory (LS) eutopic endometria obtained from women without (normal) and with endometriosis, respectively. (-) PCR amplification without template. *p < 0.05 vs. normal endometria. #p < 0.05 vs. proliferative phase.
Similar articles
- Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis.
Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Meresman GF, et al. Fertil Steril. 2000 Oct;74(4):760-6. doi: 10.1016/s0015-0282(00)01522-3. Fertil Steril. 2000. PMID: 11020520 - Apoptosis and Ki-67 expression in adenomyotic lesions and in the corresponding eutopic endometrium.
Matsumoto Y, Iwasaka T, Yamasaki F, Sugimori H. Matsumoto Y, et al. Obstet Gynecol. 1999 Jul;94(1):71-7. doi: 10.1016/s0029-7844(99)00279-3. Obstet Gynecol. 1999. PMID: 10389721 - Anti-apoptotic and pro-apoptotic gene expression evaluated from eutopic endometrium in the proliferative phase of the menstrual cycle among women with endometriosis and healthy controls.
Zubor P, Hatok J, Galo S, Dokus K, Klobusiakova D, Danko J, Racay P. Zubor P, et al. Eur J Obstet Gynecol Reprod Biol. 2009 Aug;145(2):172-6. doi: 10.1016/j.ejogrb.2009.04.024. Epub 2009 May 20. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19467764 - Phenotypic and functional studies of leukocytes in human endometrium and endometriosis.
Jones RK, Bulmer JN, Searle RF. Jones RK, et al. Hum Reprod Update. 1998 Sep-Oct;4(5):702-9. doi: 10.1093/humupd/4.5.702. Hum Reprod Update. 1998. PMID: 10027623 Review. - Apoptosis in human endometrium and endometriosis.
Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E, Terakawa N. Harada T, et al. Hum Reprod Update. 2004 Jan-Feb;10(1):29-38. doi: 10.1093/humupd/dmh007. Hum Reprod Update. 2004. PMID: 15005462 Review.
Cited by
- Endometriosis Increases the Risk of Placenta Previa in Both IVF Pregnancies and the General Obstetric Population.
Gómez-Pereira E, Burgos J, Mendoza R, Pérez-Ruiz I, Olaso F, García D, Malaina I, Matorras R. Gómez-Pereira E, et al. Reprod Sci. 2023 Mar;30(3):854-864. doi: 10.1007/s43032-022-01054-2. Epub 2022 Aug 23. Reprod Sci. 2023. PMID: 35999442 - A baboon model for endometriosis: implications for fertility.
Hastings JM, Fazleabas AT. Hastings JM, et al. Reprod Biol Endocrinol. 2006;4 Suppl 1(Suppl 1):S7. doi: 10.1186/1477-7827-4-S1-S7. Reprod Biol Endocrinol. 2006. PMID: 17118171 Free PMC article. Review. - Dysregulated sphingolipid metabolism in endometriosis.
Lee YH, Tan CW, Venkatratnam A, Tan CS, Cui L, Loh SF, Griffith L, Tannenbaum SR, Chan JK. Lee YH, et al. J Clin Endocrinol Metab. 2014 Oct;99(10):E1913-21. doi: 10.1210/jc.2014-1340. Epub 2014 Jun 24. J Clin Endocrinol Metab. 2014. PMID: 24960545 Free PMC article. - Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease.
Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Flores I, et al. Fertil Steril. 2007 May;87(5):1180-99. doi: 10.1016/j.fertnstert.2006.07.1550. Fertil Steril. 2007. PMID: 17478174 Free PMC article. - Persistent dysregulation of genes in the development of endometriosis.
Chen Y, Ma Y, Zhai Y, Yang H, Zhang C, Lu Y, Wei W, Cai Q, Ding X, Lu S, Fang Z. Chen Y, et al. Ann Transl Med. 2022 Nov;10(21):1175. doi: 10.21037/atm-22-4806. Ann Transl Med. 2022. PMID: 36467354 Free PMC article.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. - PubMed
- Fujii S. Secondary mullerian system and endometriosis. Am J Obstet Gynecol. 1991;165:219–225. - PubMed
- Mashburn PB, Arici M, Casey L. Expression of transforming growth factor-β messenger ribonucleic acid and the modulation of deoxyribonucleic acid synthesis by transforming growth factor-β1 in human endometrial cell. Am J Obstet Gynecol. 1994;170:1152–1158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous