Complex cardiac Nkx2-5 gene expression activated by noggin-sensitive enhancers followed by chamber-specific modules - PubMed (original) (raw)
Complex cardiac Nkx2-5 gene expression activated by noggin-sensitive enhancers followed by chamber-specific modules
Xuan Chi et al. Proc Natl Acad Sci U S A. 2005.
Abstract
We previously reported that an Nkx2-5-GFP bacterial artificial chromosome in transgenic mice recapitulated the endogenous gene activity in the heart. Here, we identified three additional previously uncharacterized distal enhancer modules of Nkx2-5: UH6, which directed transgene expression in the right ventricle, interventricular septum, and atrial ventricular canal; UH5, which directed expression in both atria; and UH4, which directed transgene expression in tongue muscle. Nkx2-5 enhancers drive cardiogenic gene activity from the earliest progenitors to the late-stage embryonic heart, reside within its 27 kb of 5' flanking sequences, organized in a tandem array. Nkx2-5 enhancers involved with stomach-, tongue-, and chamber-restricted expression displayed lacZ transgene activity and chromatin histone acetylation patterns consistent with tissue-specific expression. An examination of Nkx2-5 gene activity in murine embryonic stem cells converted to beating embryoid bodies showed that only the proximal active region 2 and GATA-Smad enhancers were chromatin-remodeled. Chromatin remodeling of active region 2 and GATA-Smad enhancers were blunted by noggin coexpression, which indicated dependence on bone morphogenetic protein signaling for their chromatin activation during activation of Nkx2-5 expression.
Figures
Fig. 1.
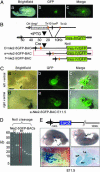
Generation of nested deletions of Nkx2-5-GFP BAC identified the most distal 5′ border of the cardiac regulatory locus. (A) A green fluorescent image merged with a brightfield image of the Nkx2-5-GFP-BAC mouse embryo. (B) The strategy as a schematic diagram for generating gap deletions by retrofitting a loxP site by transposition into the Nkx2-5-GFP-BAC. Deletions were generated by Cre recombinase between the endogenous loxP site in the BAC vector and the transposed loxP site. (C) The expression pattern of e-Nkx2-5GFP-BAC transgenic mouse line. Shown are brightfield (a), green fluorescent (b), and merged (c) images of the left ventral view and the brightfield (d) green fluorescent (e), and merged (f) images of the right ventral view of an e-Nkx2-5-GFP-BAC transgenic embryo at E11.5, showing GFP expression in the left atrium (LA) and left ventricle (LV). (D) The series of gap deletion constructs run in a pulsed-field gel electrophoresis. The e-Nkx2-5-GFP-BAC construct (which has 27 kb on the 5′ flanking sequences and 60 kb on the 3′ flanking sequences) was selected for microinjection to make transgenic mouse lines. (E) A schematic diagram of Nkx2-5-LacZ-BAC construct, which has 16 kb on the 5′ flanking sequences and 180 kb on the 3′ flanking sequences. LacZ expression was observed in Nkx2-5-LacZ-BAC mice in the right ventricle (RV) and LV but was absent in both atria. LacZ expression also was observed in the distal stomach region. RA, right atrium.
Fig. 2.
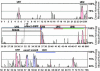
Pairwise alignment of the mouse and human Nkx2-5/Csx loci revealed multiple upstream noncoding regions of high homology. Conserved sequences are shown relative to their position in the mouse (horizontal axes), and their percent cross-homology between mouse and human sequences (50-100%) are indicated on the vertical axes. The location of the gene texas is indicated by double underlines. Horizontal arrows indicate the direction of transcription for each gene. Peaks representing the noncoding sequences (red) fitting the criteria for conserved elements as well as coding sequences (blue) meeting the percentage criteria over their entire length are indicated. UH, upstream homology region. The arrow indicates the end of the eNkx2-5-GFP deletion construct on the 5′ flanking region. UH4, UH5, and UH6 above the profile indicate the location of the fragments for transgenic analysis.
Fig. 3.
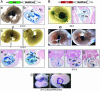
Transgenic analysis of the conserved noncoding regions functioned as previously uncharacterized enhancers for expression in the interventricular septum (IVS), atrial ventricular canal, and atria in mouse embryos. (A) Whole-mount X-gal staining (a) and transverse sections (b)of an E10.5 embryo carrying the UH6-Hsp68lacZ transgene are shown. LacZ expression was observed uniformly in the entire RV and in a subset of cells in the trabecular layer and septal wall of the LV. There was a cluster of LacZ-positive myocardial cells in the IVS and in the atrioventricular canal (AVC). (c and d) Wholemount X-gal staining (c) and transverse sections (d) of an E12.5 embryo carrying the UH6-Hsp68lacZ transgene are shown. Transgene expression was similar as that in E10.5. (B) Whole-mount X-gal staining (a) and transverse sections (b) of an E9.5 embryo carrying the UH5-Hsp68lacZ transgene, in which the LacZ expression is homogenous in the looping heart at E9.5. LacZ also is expressed in the foregut at this stage (as indicated by an arrow in a). Bb shows the transverse section of the embryo in a stained with nuclear fast red, revealing LacZ expression in the aortic sac, the common atria, and the common ventricle. Bc (right ventral view) and Bd (left ventral view) show the whole-mount staining at E12.5, with LacZ expression restricted to the RA and LA, AVC, IVS, and a subset of cells in the LV, also in the distal stomach region (as indicated by an arrow in d). _Be_-Bg show the transverse sections of the embryo in c. (C) In situ hybridization using antisense Bmp-2 mRNA on E11.5 embryonic heart, showing the intense staining in the myocardial layer of the AVC. Ca is the whole-heart view, and Cb is a heart cut in the AVC region and viewed from the above.
Fig. 4.
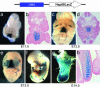
Identification of the tongue enhancer identified by UH4-Hsp68lacZ transgene expression patterns. Whole-mount X-gal staining (a, c, and _e_-g) and transverse sections (b, d, and h) of embryos carrying the UH4-Hsp68lacZNkx2-5 transgene. Transgene expression was observed specifically in the tongue primordia at E11.5 (a and b) and in the tongue muscle at E12.5 (c and d) and after (_e_-h). LacZ staining also was seen after E12.5 in the somites (f, small arrow) and the muscle in the limb (f, arrowhead).
Fig. 5.
Histone H4 acetylation patterns correlated well with temporal and spatial activity of Nkx2-5 distal enhancers and the activation of the proximal AR2 and G-S modules during ES cell induced cardiogenesis. (A) The anti-acetylhistone H4 pattern of Nkx2-5 enhancers from embryonic and neonatal tissues. (B) The RT-PCR analysis of ES cells and embryoid bodies at different time points after aggregation, with and without noggin. (C) The histone H4 acetylation pattern of AB2.2 ES cells and embryoid bodies at days 6 and 8 of differentiation. pNoggin-CS2+ expression plasmid was transfected into ES cells to block BMP signaling. pCS2+ vector served as a transfection control. (D) Whole-mount X-gal staining of an E7.5 embryo carrying the G-SHsp68lacZ transgene and a schematic diagram of the enhancers assayed by ChIP for histone acetylation patterns.
Fig. 6.
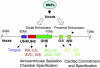
A general model for murine embryonic Nkx2-5 transcriptional regulation. _Nkx2-_5 is driven by two groups of enhancers: proximal and distal, both of which are downstream of the BMP-Smad signaling pathway. Earlier on, at the cardiac crescent stages, secreted BMPs from pharyngeal endoderm stimulate Nkx2-5 gene transcription by chromatin remodeling of the proximal enhancers G-S and AR2 in the cardiac crescent (CC) and are required for cardiac commitment and specification. The G-S enhancer is not active in later stages of heart formation, whereas AR2 provides RV expression. Later, during chamber specification, enriched BMP-Smad signaling may activate the distal enhancers, UH5 and UH6, that direct Nkx2-5 gene activity in the atria, ventricles, IVS, and AVC during chamberization.
Similar articles
- The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer.
Brown CO 3rd, Chi X, Garcia-Gras E, Shirai M, Feng XH, Schwartz RJ. Brown CO 3rd, et al. J Biol Chem. 2004 Mar 12;279(11):10659-69. doi: 10.1074/jbc.M301648200. Epub 2003 Dec 8. J Biol Chem. 2004. PMID: 14662776 - Complex modular cis-acting elements regulate expression of the cardiac specifying homeobox gene Csx/Nkx2.5.
Tanaka M, Wechsler SB, Lee IW, Yamasaki N, Lawitts JA, Izumo S. Tanaka M, et al. Development. 1999 Apr;126(7):1439-50. doi: 10.1242/dev.126.7.1439. Development. 1999. PMID: 10068637 - Direct activation of a GATA6 cardiac enhancer by Nkx2.5: evidence for a reinforcing regulatory network of Nkx2.5 and GATA transcription factors in the developing heart.
Molkentin JD, Antos C, Mercer B, Taigen T, Miano JM, Olson EN. Molkentin JD, et al. Dev Biol. 2000 Jan 15;217(2):301-9. doi: 10.1006/dbio.1999.9544. Dev Biol. 2000. PMID: 10625555 - Rb and LEK1: a "pas de deux" in cardiogenesis.
Pucéat M. Pucéat M. Cell Cycle. 2005 Aug;4(8):1030-2. doi: 10.4161/cc.4.8.1905. Epub 2005 Aug 7. Cell Cycle. 2005. PMID: 16082204 Review. - Building the heart piece by piece: modularity of cis-elements regulating Nkx2-5 transcription.
Schwartz RJ, Olson EN. Schwartz RJ, et al. Development. 1999 Oct;126(19):4187-92. doi: 10.1242/dev.126.19.4187. Development. 1999. PMID: 10477287 Review.
Cited by
- Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation.
Cofiño-Fabres C, Passier R, Schwach V. Cofiño-Fabres C, et al. Biomedicines. 2023 Aug 23;11(9):2355. doi: 10.3390/biomedicines11092355. Biomedicines. 2023. PMID: 37760796 Free PMC article. Review. - Context dependent function of APPb enhancer identified using enhancer trap-containing BACs as transgenes in zebrafish.
Shakes LA, Malcolm TL, Allen KL, De S, Harewood KR, Chatterjee PK. Shakes LA, et al. Nucleic Acids Res. 2008 Nov;36(19):6237-48. doi: 10.1093/nar/gkn628. Epub 2008 Oct 1. Nucleic Acids Res. 2008. PMID: 18832376 Free PMC article. - Coordinate Nodal and BMP inhibition directs Baf60c-dependent cardiomyocyte commitment.
Cai W, Albini S, Wei K, Willems E, Guzzo RM, Tsuda M, Giordani L, Spiering S, Kurian L, Yeo GW, Puri PL, Mercola M. Cai W, et al. Genes Dev. 2013 Nov 1;27(21):2332-44. doi: 10.1101/gad.225144.113. Genes Dev. 2013. PMID: 24186978 Free PMC article. - Generating libraries of iTol2-end insertions at BAC ends using loxP and lox511 Tn10 transposons.
Shakes LA, Abe G, Eltayeb MA, Wolf HM, Kawakami K, Chatterjee PK. Shakes LA, et al. BMC Genomics. 2011 Jul 7;12:351. doi: 10.1186/1471-2164-12-351. BMC Genomics. 2011. PMID: 21736732 Free PMC article. - GATA6 reporter gene reveals myocardial phenotypic heterogeneity that is related to variations in gap junction coupling.
Rémond MC, Iaffaldano G, O'Quinn MP, Mezentseva NV, Garcia V, Harris BS, Gourdie RG, Eisenberg CA, Eisenberg LM. Rémond MC, et al. Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1952-64. doi: 10.1152/ajpheart.00635.2011. Epub 2011 Sep 9. Am J Physiol Heart Circ Physiol. 2011. PMID: 21908788 Free PMC article.
References
- Lints, T. J., Parsons, L. M., Hartley, L., Lyons, I. & Harvey, R. P. (1993) Development (Cambridge, U.K.) 119, 419-431. - PubMed
- Bodmer, R. (1993) Development (Cambridge, U.K.) 118, 719-729. - PubMed
- Azpiazu, N. & Frasch, M. (1993) Genes Dev. 7, 1325-4130. - PubMed
- Harvey, R. P. (1996) Dev. Biol. 178, 203-216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL049953/HL/NHLBI NIH HHS/United States
- 1P20 MD00175-01/MD/NIMHD NIH HHS/United States
- 1U56 CA92077-01/CA/NCI NIH HHS/United States
- P01 HL49953/HL/NHLBI NIH HHS/United States
- P01 HL067155/HL/NHLBI NIH HHS/United States
- SO 608049/SO/PHSPO CDC HHS/United States
- U56 CA092077/CA/NCI NIH HHS/United States
- P20 MD000175/MD/NIMHD NIH HHS/United States
LinkOut - more resources
Full Text Sources