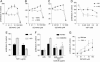Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner - PubMed (original) (raw)
Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner
Jeff S Isenberg et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Redox signaling plays an important role in the positive regulation of angiogenesis by vascular endothelial growth factor, but its role in signal transduction by angiogenesis inhibitors is less clear. Using muscle explants in 3D culture, we found that explants from mice lacking the angiogenesis inhibitor thrombospondin-1 (TSP1) exhibit exaggerated angiogenic responses to an exogenous NO donor, which could be reversed by providing exogenous TSP1. To define the basis for inhibition by TSP1, we examined the effects of TSP1 on several proangiogenic responses of endothelial cells to NO. NO has a biphasic effect on endothelial cell proliferation. The positive effect at low doses of NO is sensitive to inhibition of cGMP signaling and picomolar concentrations of TSP1. NO stimulates both directed (chemotactic) and random (chemokinetic) motility of endothelial cells in a cGMP-dependent manner. TSP1 potently inhibits chemotaxis stimulated by NO. Low doses of NO also stimulate adhesion of endothelial cells on type I collagen in a cGMP-dependent manner. TSP1 potently inhibits this response both upstream and downstream of cGMP. NO-stimulated endothelial cell responses are inhibited by recombinant type 1 repeats of TSP1 and a CD36 agonist antibody but not by the N-terminal portion of TSP1, suggesting that CD36 or a related receptor mediates these effects. These results demonstrate a potent antagonism between TSP1 and proangiogenic signaling downstream of NO. Further elucidation of this inhibitory signaling pathway may identify new molecular targets to regulate pathological angiogenesis.
Figures
Fig. 1.
Extracellular matrix invasion by vascular cells is modulated by NO and endogenous TSP1. (A_–_D) WT (A and C) and TSP1–/– (B and D) mouse muscle fragments were embedded in 3D collagen matrices and examined after 10 d either untreated (A and B) or treated with 100 μM DETA/NO (C and D). (Scale bars: 250 μm.) (E and F) Explants were treated with 1–1,000 μM DETA/NO (E) or 10 μM DETA/NO in the presence or absence of exogenous TSP1 (2.2 nM) (F). Vascular cell invasion of collagen matrices was quantified as the distance of farthest cell invasion from the muscle border in each of four quadrants. All experiments represent the mean ± SD of at least three separate experiments.
Fig. 2.
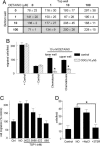
Biphasic effects of NO on proliferation of endothelial cells are modulated by exogenous and endogenous TSP1. (A) BAECs at 1,500 cells per well in DMEM supplemented with 2.5% FCS were incubated for 72 h in the presence of the indicated concentrations of DETA/NO with or without 10 μM ODQ. (B and C) HUVECs (B) or HDMVECs (C) were cultured in EGM or EGM-2MV, respectively, with 5% FCS and the indicated concentrations of DETA/NO with or without 10 μM ODQ. (D) BAECs were cultured with the indicated concentrations of TSP1 and 10 μM DETA/NO. (E and F) HDMVECs (E) and HUVECs (F) were incubated in the presence of TSP1 with or without DETA/NO or 10 μM DETA/NO with or without CD36 antibody (0.01–0.1 μg/ml). (G) Proliferation of WT and TSP1 null mouse lung endothelial cells was determined in the presence of the indicated concentrations of DETA/NO. Results represent the mean ± SD of three separate experiments.
Fig. 3.
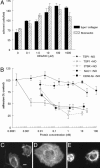
NO-stimulated endothelial cell adhesion to extracellular matrix is sGC-dependent and abrogated by TSP1. (A) HUVEC (2 × 105 cells per ml) attachment was determined on surfaces coated with type I collagen (5 μg/ml) or fibronectin (10 μg/ml). After incubating 1 h in the presence of indicated concentrations of DEA/NO, cells were fixed, stained, and counted. (B) Adhesion of DEA/NO-treated endothelial cells was analyzed in the presence of the indicated concentrations of exogenous TSP1, 3TSR, NoC1, and CD36 antibody with results normalized to maximum response under DEA/NO stimulation (10 μM). All results are presented as the mean ± SD of at least three separate experiments. (C_–_E) Untreated HUVECs (C) or HUVECs in the presence of 10 μM DEA/NO (D) or DEA/NO plus 220 pM TSP1 (E) were incubated on a collagen substrate for 60 min and then fixed and stained with phalloidin to visualize F actin. (Scale bar: 10 μm.)
Fig. 4.
TSP1 inhibits endothelial cell migration to NO via its type 1 repeats. (A) HUVEC chemotaxis and chemokinesis were assessed in modified Boyden chambers. Migration was induced by DETA/NO at the indicated concentrations in the lower or upper chamber. Cells (0.3–0.5 × 105 per well) added to the upper chamber were allowed to migrate for 5.5 h at 37°C in 5% CO2. Results are presented as the number of cells migrated per field ± SD. (B) Cells were treated under chemotactic (10 μM DETA/NO in lower well) or chemokinetic conditions (100 μM in upper well) with or without 10 μM ODQ. (C) Chemotaxis of unstimulated cells (control) or cells toward 10 μM DEA/NO in the lower chamber was assessed in the presence of the indicated concentrations of TSP1 added in the upper chamber. (D) Cells were treated with 1 nM of the recombinant TSP1 fragments NoC1 and 3TSR, and cell migration to 10 μM DETA/NO was determined. Results are from at least three separate experiments.
Fig. 5.
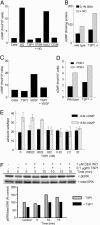
TSP1 inhibits NO-induced cGMP synthesis and signaling downstream of cGMP in endothelial cells. (A) HUVECs (5 × 103 per well) were weaned from EGM plus 5% FCS to 0.1% BSA as described in Supporting Text and treated with 10 μM DEA/NO for 5 min in the absence or presence of 100 pM of TSP1, 3TSR, NoC1, or CD36 antibody. Mean intracellular cGMP levels from duplicate determinations are presented for a representative experiment. (B) Intracellular cGMP was determined for weaned unstimulated TSP1 null and WT lung endothelial cells and cells treated for 5 min with 10 μM DEA/NO. (C) Intracellular cGMP was analyzed in weaned HUVECs after treatment with 1 μg/ml TSP1 with or without VEGF (30 ng/ml) for 10 min. (D) WT and TSP1 null lung-derived endothelial cells (5,000 cells per well) were incubated for 30 min in EGM plus 0.1% BSA with or without inhibitors of cGMP PDEs [PDE1 (25 μM 8-methoxymethyl-3-isobutyl-1-methylxanthine], PDE2 (20 nM _erythro_-9-[3-(2-hydroxynonyl)]adenine·HCl), and PDE5 [1 μM 4-{[3′,4′-(methylenedioxy)-benzyl}amoni)-6-methoxyquinazoline)], and intracellular cGMP levels were determined. (E) Modulation of endothelial cell adhesion to 5 μg/ml type I collagen was tested with 10 μM 8Br-cGMP stimulation with or without TSP1. Results are from three separate experiments. (F) HUVECs were harvested 0, 5, and 15 min after addition of 1 μM DEA/NO in medium containing 5% serum, brain extract, and heparin. Cell lysates were resolved on SDS gels and blotted with anti-ERK and phospho-ERK (pERK).
Similar articles
- Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1.
Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Ridnour LA, et al. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13147-52. doi: 10.1073/pnas.0502979102. Epub 2005 Sep 2. Proc Natl Acad Sci U S A. 2005. PMID: 16141331 Free PMC article. - Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses.
Isenberg JS, Wink DA, Roberts DD. Isenberg JS, et al. Cardiovasc Res. 2006 Sep 1;71(4):785-93. doi: 10.1016/j.cardiores.2006.05.024. Epub 2006 May 27. Cardiovasc Res. 2006. PMID: 16820142 - Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo.
Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD. Calzada MJ, et al. Circ Res. 2004 Mar 5;94(4):462-70. doi: 10.1161/01.RES.0000115555.05668.93. Epub 2003 Dec 29. Circ Res. 2004. PMID: 14699013 - Thrombospondin-1, PECAM-1, and regulation of angiogenesis.
Sheibani N, Frazier WA. Sheibani N, et al. Histol Histopathol. 1999 Jan;14(1):285-94. doi: 10.14670/HH-14.285. Histol Histopathol. 1999. PMID: 9987673 Review. - Nitric oxide and its gatekeeper thrombospondin-1 in tumor angiogenesis.
Roberts DD, Isenberg JS, Ridnour LA, Wink DA. Roberts DD, et al. Clin Cancer Res. 2007 Feb 1;13(3):795-8. doi: 10.1158/1078-0432.CCR-06-1758. Clin Cancer Res. 2007. PMID: 17289869 Review.
Cited by
- Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow.
Rogers NM, Roberts DD, Isenberg JS. Rogers NM, et al. Ann Surg. 2013 Jul;258(1):184-91. doi: 10.1097/SLA.0b013e31827e52e1. Ann Surg. 2013. PMID: 23275312 Free PMC article. - Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase.
Miller TW, Isenberg JS, Roberts DD. Miller TW, et al. Br J Pharmacol. 2010 Apr;159(7):1542-7. doi: 10.1111/j.1476-5381.2009.00631.x. Epub 2010 Mar 3. Br J Pharmacol. 2010. PMID: 20233213 Free PMC article. - Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer.
Roberts DD, Kaur S, Isenberg JS. Roberts DD, et al. Antioxid Redox Signal. 2017 Oct 20;27(12):874-911. doi: 10.1089/ars.2017.7140. Epub 2017 Sep 8. Antioxid Redox Signal. 2017. PMID: 28712304 Free PMC article. - Thrombospondins: Conserved mediators and modulators of metazoan extracellular matrix.
Adams JC. Adams JC. Int J Exp Pathol. 2024 Oct;105(5):136-169. doi: 10.1111/iep.12517. Epub 2024 Sep 12. Int J Exp Pathol. 2024. PMID: 39267379 Review. - The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1.
Meijles DN, Sahoo S, Al Ghouleh I, Amaral JH, Bienes-Martinez R, Knupp HE, Attaran S, Sembrat JC, Nouraie SM, Rojas MM, Novelli EM, Gladwin MT, Isenberg JS, Cifuentes-Pagano E, Pagano PJ. Meijles DN, et al. Sci Signal. 2017 Oct 17;10(501):eaaj1784. doi: 10.1126/scisignal.aaj1784. Sci Signal. 2017. PMID: 29042481 Free PMC article.
References
- Maulik, N. (2002) Antioxid. Redox Signal. 4, 783–784. - PubMed
- Morbidelli, L., Donnini, S., Chillemi, F., Giachetti, A. & Ziche, M. (2003) Clin. Cancer Res. 9, 5358–5369. - PubMed
- Zaragoza, C., Soria, E., Lopez, E., Browning, D., Balbin, M., Lopez-Otin, C. & Lamas, S. (2002) Mol. Pharmacol. 62, 927–935. - PubMed
- Raj, U. & Shimoda, L. (2002) Am. J. Physiol. 283, L671–L677. - PubMed
- Oliveira, C. J., Schindler, F., Ventura, A. M., Morais, M. S., Arai, R. J., Debbas, V., Stern, A. & Monteiro, H. P. (2003) Free Radical Biol. Med. 35, 381–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous