Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms - PubMed (original) (raw)
Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms
Kerry L Tomlin et al. Appl Environ Microbiol. 2005 Sep.
Abstract
Biofilm formation in Burkholderia cenocepacia has been shown to rely in part on acylhomoserine lactone-based quorum sensing. For many other bacterial species, it appears that both the initial adherence and the later stages of biofilm maturation are affected when quorum sensing pathways are inhibited. In this study, we examined the effects of mutations in the cepIR and cciIR quorum-sensing systems of Burkholderia cenocepacia K56-2 with respect to biofilm attachment and antibiotic resistance. We also examined the role of the cepIR system in biofilm stability and structural development. Using the high-throughput MBEC assay system to produce multiple equivalent biofilms, the biomasses of both the cepI and cepR mutant biofilms, measured by crystal violet staining, were less than half of the value observed for the wild-type strain. Attachment was partially restored upon providing functional gene copies via multicopy expression vectors. Surprisingly, neither the cciI mutant nor the double cciI cepI mutant was deficient in attachment, and restoration of the cciI gene resulted in less attachment than for the mutants. Meanwhile, the cciR mutant did show a significant reduction in attachment, as did the cciR cepIR mutant. While there was no change in antibiotic susceptibility with the individual cepIR and cciIR mutants, the cepI cciI mutant biofilms were more sensitive to ciprofloxacin. A significant increase in sensitivity to removal by sodium dodecyl sulfate was seen for the cepI and cepR mutants. Flow cell analysis of the individual cepIR mutant biofilms indicated that they were both structurally and temporally impaired in attachment and development. These results suggest that biofilm structural defects might be present in quorum-sensing mutants of B. cenocepacia that affect the stability and resistance of the adherent cell mass, providing a basis for future studies to design preventative measures against biofilm formation in this species, an important lung pathogen of cystic fibrosis patients.
Figures
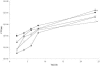
FIG. 1.
Biofilm growth curves of wild-type B. cenocepacia K56-2 (♦), K56-I2 (⋄), K56-R2 (○), K56-I2(pSLS225) (▪), and K56-R2(pSLR100) (▴). Data points represent the mean (± standard error of the mean) CFU/peg of four pegs over two separate trials (two pegs per trial) every 2 h for 8 h, with a final biofilm density count at 24 h. Significant differences in 24-h biofilm counts were observed between K56-R2 and its complemented counterpart K56-R2(pSLR100) (a, P < 0.05 by ANOVA and Tukey test).
FIG. 2.
Quantification of cellular matter of the quorum-sensing mutants and the complemented mutants represented by absorbance at 540 nm of crystal violet stain bound to 24-h biofilms cultured on the MBEC assay system. The values shown are the means ± standard errors of the means from three trials, assessing at least five pegs per strain per trial. The asterisks indicate strains that produced significantly less biomass than K56-2 (P < 0.05 by ANOVA and Dunn test). K56-R2(pSLR100) produced significantly greater biomass than K56-R2(pUCP28T) (a), and K56-2 cciI(pRM164) produced significantly less biomass than K56-2 cciI(pUCP26) (b) (P < 0.01 by ANOVA and Dunn test).
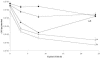
FIG. 3.
Comparative analysis of cell detachment of B. cenocepacia K56-2 (♦), K56-I2 (⋄), K56-R2 (○), K56-I2(pSLS225) (▪), and K56-R2(pSLR100) (▴) during a 24-hour treatment with 0.2% (wt/vol) SDS. Data points represent the mean (± standard error of the mean) CFU/peg of four pegs over two separate trials (two pegs per trial) every 2 h for 8 h, with a final biofilm density count at 24 h. By the end of the 24-hour time course, there were significantly fewer cells attached in the K56-I2 and K56-R2 biofilms than in those of the wild-type K56-2 and complemented K56-I2(pSLS225) and K56-R2(pSLR100) strains (a and b, P < 0.05 by ANOVA and Tukey test).
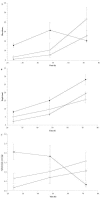
FIG. 4.
Characteristics of glass coverslip flow cell biofilms of B. cenocepacia K56-2 pHKT2 (♦), K56-I2 pHKT1 (⋄), and K56-R2 pHKT2 (○) as assessed by deconvolution epifluorescence microscopy. A) Biovolume was defined as the average percent area of active pixels over the slices of the stack multiplied by the stack depth (micrometers). B) Stack depth was defined as the number of slices in the stack multiplied by the slice depth (micrometers). C) Substratum coverage was defined as the percent area of active pixels recorded in the slice at the coverslip surface. All data points represent the means ± standard errors of the means from at least three separate trials, with three image stacks captured per time point per trial.
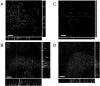
FIG. 5.
Deconvolved epifluorescence micrographs (stack average) of B. cenocepacia K56-2(pHKT2) flow cell biofilms at A) 24 h and B) 72 h and of B. cenocepacia K56-R2(pHKT2) flow cell biofilms at C) 24 h and D) 72 h. Slices were taken at 1.0-mm _z_-plane intervals using a Leica DMR epifluorescence microscope equipped with a focus motor at an exposure of 1.0 seconds and were volume deconvolved using Openlab 3.0.9 software. Side panels represent 1.0-μm slices in the x and y planes. Bars, 10 μm.
Similar articles
- Characterization of the cciIR quorum-sensing system in Burkholderia cenocepacia.
Malott RJ, Baldwin A, Mahenthiralingam E, Sokol PA. Malott RJ, et al. Infect Immun. 2005 Aug;73(8):4982-92. doi: 10.1128/IAI.73.8.4982-4992.2005. Infect Immun. 2005. PMID: 16041013 Free PMC article. - Identification of genes regulated by the cepIR quorum-sensing system in Burkholderia cenocepacia by high-throughput screening of a random promoter library.
Subsin B, Chambers CE, Visser MB, Sokol PA. Subsin B, et al. J Bacteriol. 2007 Feb;189(3):968-79. doi: 10.1128/JB.01201-06. Epub 2006 Nov 22. J Bacteriol. 2007. PMID: 17122351 Free PMC article. - Reciprocal regulation by the CepIR and CciIR quorum sensing systems in Burkholderia cenocepacia.
O'Grady EP, Viteri DF, Malott RJ, Sokol PA. O'Grady EP, et al. BMC Genomics. 2009 Sep 17;10:441. doi: 10.1186/1471-2164-10-441. BMC Genomics. 2009. PMID: 19761612 Free PMC article. - Social interactions in the Burkholderia cepacia complex: biofilms and quorum sensing.
Coenye T. Coenye T. Future Microbiol. 2010 Jul;5(7):1087-99. doi: 10.2217/fmb.10.68. Future Microbiol. 2010. PMID: 20632807 Review. - Quorum sensing systems influence Burkholderia cenocepacia virulence.
Subramoni S, Sokol PA. Subramoni S, et al. Future Microbiol. 2012 Dec;7(12):1373-87. doi: 10.2217/fmb.12.118. Future Microbiol. 2012. PMID: 23231487 Review.
Cited by
- Control of Biofilm Formation in Healthcare: Recent Advances Exploiting Quorum-Sensing Interference Strategies and Multidrug Efflux Pump Inhibitors.
Subhadra B, Kim DH, Woo K, Surendran S, Choi CH. Subhadra B, et al. Materials (Basel). 2018 Sep 10;11(9):1676. doi: 10.3390/ma11091676. Materials (Basel). 2018. PMID: 30201944 Free PMC article. Review. - Competitive Fitness of Essential Gene Knockdowns Reveals a Broad-Spectrum Antibacterial Inhibitor of the Cell Division Protein FtsZ.
Hogan AM, Scoffone VC, Makarov V, Gislason AS, Tesfu H, Stietz MS, Brassinga AKC, Domaratzki M, Li X, Azzalin A, Biggiogera M, Riabova O, Monakhova N, Chiarelli LR, Riccardi G, Buroni S, Cardona ST. Hogan AM, et al. Antimicrob Agents Chemother. 2018 Nov 26;62(12):e01231-18. doi: 10.1128/AAC.01231-18. Print 2018 Dec. Antimicrob Agents Chemother. 2018. PMID: 30297366 Free PMC article. - Loss of _O_-Linked Protein Glycosylation in Burkholderia cenocepacia Impairs Biofilm Formation and Siderophore Activity and Alters Transcriptional Regulators.
Oppy CC, Jebeli L, Kuba M, Oates CV, Strugnell R, Edgington-Mitchell LE, Valvano MA, Hartland EL, Newton HJ, Scott NE. Oppy CC, et al. mSphere. 2019 Nov 13;4(6):e00660-19. doi: 10.1128/mSphere.00660-19. mSphere. 2019. PMID: 31722994 Free PMC article. - Immune Recognition of the Epidemic Cystic Fibrosis Pathogen Burkholderia dolosa.
Roux D, Weatherholt M, Clark B, Gadjeva M, Renaud D, Scott D, Skurnik D, Priebe GP, Pier G, Gerard C, Yoder-Himes DR. Roux D, et al. Infect Immun. 2017 May 23;85(6):e00765-16. doi: 10.1128/IAI.00765-16. Print 2017 Jun. Infect Immun. 2017. PMID: 28348057 Free PMC article. - A Comparative Analysis of Acyl-Homoserine Lactone Synthase Assays.
Shin D, Frane ND, Brecht RM, Keeler J, Nagarajan R. Shin D, et al. Chembiochem. 2015 Dec;16(18):2651-9. doi: 10.1002/cbic.201500387. Epub 2015 Nov 6. Chembiochem. 2015. PMID: 26456773 Free PMC article.
References
- Al-Bakri, A. G., P. Gilbert, and D. G. Allison. 2004. Immigration and emigration of Burkholderia cepacia and Pseudomonas aeruginosa between and within mixed biofilm communities. J Appl. Microbiol. 96:455-463. - PubMed
- Balaban, N., A. Giacometti, O. Cirioni, Y. Gov, R. Ghiselli, F. Mocchegiani, C. Viticchi, M. S. Del Prete, V. Saba, G. Scalise, and G. Dell’Acqua. 2003. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 187:625-630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources