A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease - PubMed (original) (raw)
A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease
Walter J Lukiw et al. J Clin Invest. 2005 Oct.
Abstract
Deficiency in docosahexaenoic acid (DHA), a brain-essential omega-3 fatty acid, is associated with cognitive decline. Here we report that, in cytokine-stressed human neural cells, DHA attenuates amyloid-beta (Abeta) secretion, an effect accompanied by the formation of NPD1, a novel, DHA-derived 10,17S-docosatriene. DHA and NPD1 were reduced in Alzheimer disease (AD) hippocampal cornu ammonis region 1, but not in the thalamus or occipital lobes from the same brains. The expression of key enzymes in NPD1 biosynthesis, cytosolic phospholipase A2 and 15-lipoxygenase, was altered in AD hippocampus. NPD1 repressed Abeta42-triggered activation of proinflammatory genes while upregulating the antiapoptotic genes encoding Bcl-2, Bcl-xl, and Bfl-1(A1). Soluble amyloid precursor protein-alpha stimulated NPD1 biosynthesis from DHA. These results indicate that NPD1 promotes brain cell survival via the induction of antiapoptotic and neuroprotective gene-expression programs that suppress Abeta42-induced neurotoxicity.
Figures
Figure 1
DHA attenuates Aβ peptide secretion and serves as the precursor for NPD1 biosynthesis; meanwhile, sAPPα activates NPD1 formation. (A) HN cells were grown for up to 8 weeks. (B and C) After 4 weeks of culture, aging HN cells displayed approximately equal populations of neurons and glia and stained positive with the (red fluorescent) neuron-specific marker βIII tubulin (6) (B) and the (green fluorescent) glia-specific marker GFAP (C). (D and E) HN cells in culture normally release Aβ40 and Aβ42 peptides over 8 weeks of aging. Secretion by HN cells of Aβ42 peptide was approximately one-tenth that of Aβ40 peptide; IL-1β (10 ng/ml in modified HNMM; see ref. 3) increased, and DHA decreased, the release of both Aβ40 and Aβ42 peptides into the cell culture medium. CON, control. (F) DHA (100 nM) induced NPD1 biosynthesis in HN cells, and this induction was age-dependent. (G–I) HN cells incubated in the presence of 10, 20, 50, and 100 ng/ml of sAPPα showed dose-dependent upregulation of NPD1 formation (G); HN cells incubated with sAPPα (at 20 and 100 ng/ml) and/or DHA (at 50 nM; even in the presence of Aβ42) also displayed upregulated production of NPD1 (H and I). *P < 0.05 (ANOVA).
Figure 2
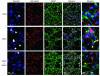
Aβ42-induced apoptosis is inhibited by NPD1 in HN cells. After 3 weeks in culture, HN cells were treated with DMEM/F12 (control), Aβ42 (8 μM) in DMEM/F12 (Aβ42), or Aβ42 (8 μM) plus NPD1 (50 nM) (Aβ42+NPD1) in DMEM/F12 for 3.5 days, then stained with Hoechst 33258 (Hoechst), anti–βIII tubulin (βIII tubulin), and anti-GFAP (GFAP), and all 3 stains were imaged together (merge A). As revealed by Hoechst staining, in controls, relatively few compact nuclei were observed; in Aβ42-treated cells, many compact nuclei were seen (white arrows). In Aβ42-treated cells, neurons and glia clumped together with condensed cytoplasm and damaged nuclei, and both βIII tubulin and GFAP staining was reduced (compare merges of control, Aβ42, and Aβ42+NPD1-treated cells). When NPD1 was added to Aβ42-treated cells, Hoechst staining revealed many fewer compacted nuclei. Each field under columns marked βIII tubulin, GFAP, and merge A represents approximately 0.1 mm2. A comparison of control, Aβ42-treated, and Aβ42+NPD1-treated HN cells at higher magnification, stained with Hoechst 33258 (blue), anti–βIII tubulin (red), and anti-GFAP (green), shows that Aβ42-induced apoptosis is associated with both neuronal and glial nuclei (merge B), and that Aβ42+NPD1-treated HN cells show significantly decreased numbers of compacted apoptotic nuclei (yellow arrows).
Figure 3
Aβ42-induced apoptosis is associated with both neurons and glia. (A) Compacted nuclei were associated with both neurons (white arrows) and glia (pink arrows), and with non–βIII tubulin– or non–GFAP-staining cells (non-neural cells; yellow arrows). (B) Apoptosis, as monitored by the presence of compacted nuclei of no more than 0.5 μm diameter (5, 6) after Hoechst 33258 staining, was significantly suppressed by NPD1. Analysis of numbers of compacted nuclei per field in both neurons (red bars) and glia (green bars) shows that these were significantly attenuated in the presence of Aβ42+NPD1 when compared with Aβ42 alone; black bars indicate abundance of compacted nuclei associated with non–βIII tubulin–staining/non–GFAP-staining cells. n = 16. *P < 0.01 and **P < 0.05 versus controls (ANOVA).
Figure 4
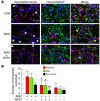
Changes in gene expression in HN cells in the presence of Aβ42 (A), DHA (B), and NPD1 (C). Truncated volcano plots display gene-expression patterns as a function of fold change over age-matched controls, against P (ANOVA). “Nonsignificant genes” that would normally appear in the region of P < 0.05 and fold change of less than 1.0 (either up- or downregulated) have been omitted for clarity. Genes of highest statistical significance are sequestered into the lower left (blue, downregulated) and lower right (red, upregulated) quadrants; further data for a select group of 10 highly significant up- and downregulated genes appear in Table 1 and in Supplemental Table 1. (D) Western immunoblot analysis confirmed upregulation of Bcl-2 and Bfl-1(A1) antiapoptotic proteins over actin controls in DHA- and NPD1-treated cells. Gene transcripts have been classified according to their known major functions, although most of these RNAs may have multiple cellular functions (33). (E) Quantified intensities of Bcl-2 and Bfl-1(A1) bands normalized to constitutively expressed actin in the same sample are shown as bar graphs. *P < 0.02 versus controls.
Figure 5
NPD1 and DHA are reduced in AD brain. (A) When compared with age-matched controls, NPD1 and unesterified DHA were significantly reduced in AD hippocampus (HIP) and temporal lobe (TEM) but not in the thalamus (THA) or occipital lobe (OCC) of the same AD brains. In AD, thalamic and occipital regions were relatively spared AD neuropathology. Signals for NPD1 and unesterified DHA in AD hippocampus averaged about one-twentieth and one-half, respectively, of those values seen in age-matched controls. LC-PDA-ESI-MS-MS–based lipidomic analysis (sensitivity 0.05 pmol/mg total protein). n = 6. *P < 0.01 (ANOVA). (B) Characterization of NPD1 using LC-PDA-ESI-MS-MS–based lipidomic analysis (5, 6). (C) Mass spectrographic identification of 10,17_S_-docosatriene (NPD1) in human hippocampus. (D) Proposed biosynthetic pathways from DHA to NPD1 and bioactivity. DHA is highly enriched as an acyl side chain of brain and retinal membrane phospholipids, suggesting its importance as an essential component of brain and retinal function (2, 29). Esterified DHA is liberated by PLA2 action upon membrane phospholipids, whereupon it is oxygenated, initially via a 15-LOX–like enzyme, into 10,17_S_-docosatriene (NPD1). sAPPα, a secreted neurotrophic peptide, stimulates NPD1 biosynthesis. NPD1 exhibits neuroprotective activity against Aβ42 action, represses apoptosis, and promotes the expression of antiapoptotic genes encoding Bcl-2 and Bfl-1(A1) (37).
Similar articles
- Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection.
Bazan NG. Bazan NG. Prostaglandins Leukot Essent Fatty Acids. 2009 Aug-Sep;81(2-3):205-11. doi: 10.1016/j.plefa.2009.05.024. Epub 2009 Jun 11. Prostaglandins Leukot Essent Fatty Acids. 2009. PMID: 19520558 Free PMC article. Review. - Survival signalling in Alzheimer's disease.
Lukiw WJ, Bazan NG. Lukiw WJ, et al. Biochem Soc Trans. 2006 Dec;34(Pt 6):1277-82. doi: 10.1042/BST0341277. Biochem Soc Trans. 2006. PMID: 17073801 Review. - Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress.
Bazan NG. Bazan NG. Brain Pathol. 2005 Apr;15(2):159-66. doi: 10.1111/j.1750-3639.2005.tb00513.x. Brain Pathol. 2005. PMID: 15912889 Free PMC article. Review. - Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress.
Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Mukherjee PK, et al. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8491-6. doi: 10.1073/pnas.0402531101. Epub 2004 May 19. Proc Natl Acad Sci U S A. 2004. PMID: 15152078 Free PMC article. - Lipid-mediated cell signaling protects against injury and neurodegeneration.
Zhang C, Bazan NG. Zhang C, et al. J Nutr. 2010 Apr;140(4):858-63. doi: 10.3945/jn.109.114884. Epub 2010 Feb 24. J Nutr. 2010. PMID: 20181788 Free PMC article.
Cited by
- Improved outcome after peripheral nerve injury in mice with increased levels of endogenous ω-3 polyunsaturated fatty acids.
Gladman SJ, Huang W, Lim SN, Dyall SC, Boddy S, Kang JX, Knight MM, Priestley JV, Michael-Titus AT. Gladman SJ, et al. J Neurosci. 2012 Jan 11;32(2):563-71. doi: 10.1523/JNEUROSCI.3371-11.2012. J Neurosci. 2012. PMID: 22238091 Free PMC article. - The Role of Eicosanoids in Alzheimer's Disease.
Biringer RG. Biringer RG. Int J Environ Res Public Health. 2019 Jul 18;16(14):2560. doi: 10.3390/ijerph16142560. Int J Environ Res Public Health. 2019. PMID: 31323750 Free PMC article. Review. - Nutrition, brain aging, and neurodegeneration.
Joseph J, Cole G, Head E, Ingram D. Joseph J, et al. J Neurosci. 2009 Oct 14;29(41):12795-801. doi: 10.1523/JNEUROSCI.3520-09.2009. J Neurosci. 2009. PMID: 19828791 Free PMC article. Review. - Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus.
Serhan CN, Chiang N. Serhan CN, et al. Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S200-15. doi: 10.1038/sj.bjp.0707489. Epub 2007 Oct 29. Br J Pharmacol. 2008. PMID: 17965751 Free PMC article. Review. - Lipid emulsions - Guidelines on Parenteral Nutrition, Chapter 6.
Adolph M, Heller AR, Koch T, Koletzko B, Kreymann KG, Krohn K, Pscheidl E, Senkal M; Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. Adolph M, et al. Ger Med Sci. 2009 Nov 18;7:Doc22. doi: 10.3205/000081. Ger Med Sci. 2009. PMID: 20049078 Free PMC article. Review.
References
- Bazan NG, Lukiw WJ. Cyclooxygenase-2 and presenilin-1 gene expression induced by interleukin-1beta and amyloid beta 42 peptide is potentiated by hypoxia in primary human neural cells. J. Biol. Chem. 2002;277:30359–30367. - PubMed
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease [review] Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:741–749. - PubMed
- Marcheselli VL, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–43817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG018031/AG/NIA NIH HHS/United States
- GM38765/GM/NIGMS NIH HHS/United States
- RR16816/RR/NCRR NIH HHS/United States
- R01 GM038765/GM/NIGMS NIH HHS/United States
- P20 RR016816/RR/NCRR NIH HHS/United States
- NS23002/NS/NINDS NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
- NS46741/NS/NINDS NIH HHS/United States
- AG18031/AG/NIA NIH HHS/United States
- R01 NS046741/NS/NINDS NIH HHS/United States
- R01 NS023002/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials