hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer - PubMed (original) (raw)
hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer
Renty B Franklin et al. Mol Cancer. 2005.
Abstract
Background: The genetic and molecular mechanisms responsible for and associated with the development and progression of prostate malignancy are largely unidentified. The peripheral zone is the major region of the human prostate gland where malignancy develops. The normal peripheral zone glandular epithelium has the unique function of accumulating high levels of zinc. In contrast, the ability to accumulate zinc is lost in the malignant cells. The lost ability of the neoplastic epithelial cells to accumulate zinc is a consistent factor in their development of malignancy. Recent studies identified ZIP1 (SLC39A1) as an important zinc transporter involved in zinc accumulation in prostate cells. Therefore, we investigated the possibility that down-regulation of hZIP1 gene expression might be involved in the inability of malignant prostate cells to accumulate zinc. To address this issue, the expression of hZIP1 and the depletion of zinc in malignant versus non-malignant prostate glands of prostate cancer tissue sections were analyzed. hZIP1 expression was also determined in malignant prostate cell lines.
Results: hZIP1 gene expression, ZIP1 transporter protein, and cellular zinc were prominent in normal peripheral zone glandular epithelium and in benign hyperplastic glands (also zinc accumulating glands). In contrast, hZIP1 gene expression and transporter protein were markedly down-regulated and zinc was depleted in adenocarcinomatous glands and in prostate intra-epithelial neoplastic foci (PIN). These changes occur early in malignancy and are sustained during its progression in the peripheral zone. hZIP1 is also expressed in the malignant cell lines LNCaP, PC-3, DU-145; and in the nonmalignant cell lines HPr-1 and BPH-1.
Conclusion: The studies clearly establish that hZIP1 gene expression is down regulated and zinc is depleted in adenocarcinomatous glands. The fact that all the malignant cell lines express hZIP1 indicates that the down-regulation in adenocarcinomatous glands is likely due to in situ gene silencing. These observations, coupled with the numerous and consistent reports of loss of zinc accumulation in malignant cells in prostate cancer, lead to the plausible proposal that down regulation of hZIP1 is a critical early event in the development prostate cancer.
Figures
Figure 1
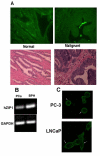
(A) Immunohistochemical determination of ZIP 1 transporter levels in normal and malignant prostate glands. The strong positive reaction is evident in the normal gland secretory epithelial cells that border the lumen, and is virtually absent in the malignant glands. Note that ZIP1 is not apparent in the stroma. (B) RT-PCR of RNA extracted from malignant prostate tissue and benign prostatic hyperplasia. Note the marked decrease in ZIP1 mRNA in the malignant tissue. Results are representative of two independent samples. Density of the bands was determined by densitometry scans and GAPDH band intensity used to normalize hZIP1 mRNA. hZIP1/GAPDH for PCa and BPH were 0.71 ± 0.067 and 1.02 ± 0.092 respectively. (C) Immunohistochemical detection of ZIP1 in malignant prostate cell lines. Note the association of ZIP1 with the plasma membrane.
Figure 2
Immunohistochemical detection of ZIP1 transporter protein in malignant and nonmalignant loci of a representative prostate cancer tissue section. (A) BPH, magnification is 1000×, bar = 10 μm. (B) Normal, magnification is 400×, bar = 25 μm. (C) PIN, magnification is 400×, bars = 25 μm. (D) Adenocarcinoma, magnification 400×, bar = 10 μm Note the immuno-positivity of the plasma membrane of BPH and normal glands. The malignant and PIN loci show no detectable ZIP1 so that the plasma membrane of these cells is not visible.
Figure 3
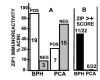
Comparative results of ZIP1 immuno-positive glands of tissue sections from subjects described in Table 1. A. Summary of glands that exhibited a positive Zip1 reactivity. The number of cases is shown in each bar. B. The number of cases in which the glandular epithelium contained cells that exhibited a ZIP1 score >+ (more than 10% of the cells comprising the acini). The differences in A and B between BPH glands and adenocarcinomatous glands are significant, P < 0.01.
Figure 4
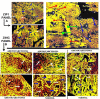
In situ detection of ZIP1 mRNA and zinc levels in normal and malignant glands. Panel A. Representative ZIP1 mRNA in Prostate Sections . Sections (inserts 1,2) from two prostate cancer subjects are shown with low magnification. Blue arrows point to acini with normal glandular epithelium that exhibit ZIP1 mRNA. White arrows point to adenocarcinomatous glands in which ZIP1 expression is not demonstrable. Insert 3 is a higher magnification of a section from a cancer patient to show more detail. Blue arrows point to acini with normal glandular epithelium. Red arrows point to malignant glands. Green arrows point to stromal (fibromuscular) tissue. The malignant epithelial cells exhibit a complete absence of detectable ZIP1 mRNA in the glandular epithelium. The normal glandular epithelium exhibits ZIP1 expression; and no ZIP1 expression in the stroma. Normal acini marked 'a' show uniform ZIP1 mRNA expression in the glandular epithelium. Advanced adenocarcinomatous glands marked as 'b" show uniform absence of ZIP1 mRNA. Developing early stage adenocarcinomatous glands marked 'c' show a progression of normal ZIP1 expressing cells and malignant cells that lost the expression of ZIP1. Panel B. Representative Zinc Levels in Prostate Sections. High zinc is represented by Newport Green yellow stain and low zinc is represented by TSQ red stain. The malignant region of the peripheral zone shows a significant depletion of zinc in the malignant glandular epithelium as exhibited by the red staining (white arrows). The depletion of zinc is evident in early differentiated malignant glands as represented by combinations of red and yellow staining in the glandular epithelial cells. As malignancy advances to the undifferentiated stage, the zinc is further depleted as represented by the dominant red stain and no yellow stain in the glandular epithelium of the adenocarcinomatous glands. The depletion of zinc in the malignant glandular region results in the surrounding stroma showing a higher zinc level (green stain) than the glandular epithelium. In contrast, the normal peripheral zone glands exhibit high zinc levels as represented by the uniform yellow stain and absence of red stain in the glandular epithelium. The stroma surrounding the glands exhibits a lower zinc level as shown by the red stain.
Figure 5
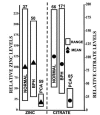
Composite of zinc and citrate levels in prostate. The zinc data are taken from Zaichick et al [9] and show the range of zinc levels in resected prostate tissue samples from different subjects. The citrate data are taken from Kurhanewicz et al [41] and show the range of citrate levels as determined by in situ magnetic resonance spectroscopy imaging of the prostate gland of different subjects. The actual zinc and citrate concentrations for normal were set to 100 and the values for BPH and PCa were adjusted accordingly. Note the parallelisms in that zinc and citrate levels are consistently significantly low in malignancy; and that no case exists in which the malignant loci retain the high zinc or high citrate levels that characterize normal or hypertrophic glands. The values above each bar are the number of subjects.
Figure 6
The integrated role of ZIP1, zinc, and citrate metabolism in the pathogenesis of prostate malignancy. The normal glandular epithelial cell expresses ZIP1 that permits zinc accumulation, which inhibits citrate oxidation and terminal respiration. Citrate accumulates and coupled ATP production is reduced. A genetic transformation results in a neoplastic cell with potential malignant capability. ZIP1 expression is silenced by epigenetic factors which eliminate Zip1 transporter and accumulation of zinc in the premalignant cell. The level of cellular zinc decreases which removes the inhibitory effects on citrate oxidation and terminal oxidation. The Krebs cycle is functional and coupled ATP production is increased. The malignant cell is metabolically and bioenergetically capable of manifesting its malignant potential. Additionally, the growth inhibitory effect of zinc is removed, which allows growth and progression of the malignant cell.
Similar articles
- hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands.
Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. Desouki MM, et al. Mol Cancer. 2007 Jun 5;6:37. doi: 10.1186/1476-4598-6-37. Mol Cancer. 2007. PMID: 17550612 Free PMC article. - Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues.
Singh KK, Desouki MM, Franklin RB, Costello LC. Singh KK, et al. Mol Cancer. 2006 Apr 4;5:14. doi: 10.1186/1476-4598-5-14. Mol Cancer. 2006. PMID: 16595004 Free PMC article. - hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1).
Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. Zou J, et al. Prostate. 2011 Oct 1;71(14):1518-24. doi: 10.1002/pros.21368. Epub 2011 Feb 25. Prostate. 2011. PMID: 21360563 Free PMC article. - The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots.
Costello LC, Franklin RB. Costello LC, et al. Mol Cancer. 2006 May 15;5:17. doi: 10.1186/1476-4598-5-17. Mol Cancer. 2006. PMID: 16700911 Free PMC article. Review. - A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer.
Costello LC, Franklin RB. Costello LC, et al. Arch Biochem Biophys. 2016 Dec 1;611:100-112. doi: 10.1016/j.abb.2016.04.014. Epub 2016 Apr 27. Arch Biochem Biophys. 2016. PMID: 27132038 Free PMC article. Review.
Cited by
- The role of the prostate in male fertility, health and disease.
Verze P, Cai T, Lorenzetti S. Verze P, et al. Nat Rev Urol. 2016 Jul;13(7):379-86. doi: 10.1038/nrurol.2016.89. Epub 2016 Jun 1. Nat Rev Urol. 2016. PMID: 27245504 Review. - In situ clinical evidence that zinc levels are decreased in breast invasive ductal carcinoma.
Costello LC, Zou J, Franklin RB. Costello LC, et al. Cancer Causes Control. 2016 Jun;27(6):729-35. doi: 10.1007/s10552-016-0746-1. Epub 2016 Apr 20. Cancer Causes Control. 2016. PMID: 27097912 Free PMC article. - Zinc mediated normalization of histoarchitecture and antioxidant status offers protection against initiation of experimental carcinogenesis.
Chadha VD, Vaiphei K, Dhawan DK. Chadha VD, et al. Mol Cell Biochem. 2007 Oct;304(1-2):101-8. doi: 10.1007/s11010-007-9490-x. Epub 2007 May 26. Mol Cell Biochem. 2007. PMID: 17530192 - A Proposed Efficacious Treatment with Clioquinol (Zinc Ionophore) and Cabergoline (Prolactin Dopamine Agonist) for the Treatment of Terminal Androgen-independent Prostate Cancer. Why and How?
Costello LC, Franklin RB. Costello LC, et al. J Clin Res Oncol. 2019;2(1):https://asclepiusopen.com/journal-of-clinical-research-in-oncology/volume-2-issue-1/1.pdf. J Clin Res Oncol. 2019. PMID: 30828702 Free PMC article. - Deciphering the role of zinc homeostasis in the tumor microenvironment and prognosis of prostate cancer.
Guo T, Wang J, Meng X, Wang Y, Lou Y, Ma J, Xu S, Ni X, Jia Z, Jin L, Wang C, Chen Q, Li P, Huang Y, Ren S. Guo T, et al. Discov Oncol. 2024 Jun 4;15(1):207. doi: 10.1007/s12672-024-01006-z. Discov Oncol. 2024. PMID: 38833013 Free PMC article.
References
- Gonic P, Oberleas D, Knechtges T, Prasad AS. Atomic absorption determination of zinc in the prostate. Invest Urol. 1969;6:345–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-097714/PHS HHS/United States
- CA 79903/CA/NCI NIH HHS/United States
- R01 CA079903/CA/NCI NIH HHS/United States
- R01 CA071207/CA/NCI NIH HHS/United States
- CA 71207/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases