Human striatal activation reflects degree of stimulus saliency - PubMed (original) (raw)
Human striatal activation reflects degree of stimulus saliency
Caroline F Zink et al. Neuroimage. 2006.
Abstract
Salient stimuli are characterized by their capability to perturb and seize available cognitive resources. Although the striatum and its dopaminergic inputs respond to a variety of stimuli categorically defined as salient, including rewards, the relationship between striatal activity and saliency is not well understood. Specifically, it is unclear if the striatum responds in an all-or-none fashion to salient events or instead responds in a graded fashion to the degree of saliency associated with an event. Using functional magnetic resonance imaging, we measured activity in the brains of 20 participants performing a visual classification task in which they identified single digits as odd or even numbers. An auditory tone preceded each number, which was occasionally, and unexpectedly, substituted by a novel sound. The novel sounds varied in their ability to interrupt and reallocate cognitive resources (i.e., their saliency) as measured by a delay in reaction time to immediately subsequent numerical task-stimuli. The present findings demonstrate that striatal activity increases proportionally to the degree to which an unexpected novel sound interferes with the current cognitive focus, even in the absence of reward. These results suggest that activity in the human striatum reflects the level of saliency associated with a stimulus, perhaps providing a signal to reallocate limited resources to important events.
Figures
Fig. 1
Schematic diagram of the experimental task. A single digit number appeared on the screen for 200 ms followed by a blank screen for 2500 ms. Participants were required to classify the number as either odd or even with a button press (shown here with button #1 corresponding to odd numbers and button #2 corresponding to even numbers). 600 ms preceding the appearance of the number, the outline of a circle appeared and remained on the screen for the duration of the trial, such that the number was presented in the center of the circle outline. Concurrent with the circle outline appearance, a 500 ms sound was played for the participants via headphones. The sound was a 600 Hz ‘‘standard’’ tone for 85% of trials, a 700 Hz ‘‘deviant’’ tone for 5% of trials, and a ‘‘novel’’ sound (e.g., a siren or a burst of noise) for 10% of the trials. The standard and deviant tones were considered minimally salient. The novel sounds were designed to be associated with varying degrees of saliency.
Fig. 2
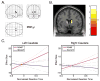
Brain regions where a correlation between BOLD activity and reaction times (to subsequent numerical task-stimuli) was significantly greater for the novel sounds compared to deviant tones. The only significant activations (P < 0.001 uncorrected; voxel extent = 10) were in the bilateral caudate (A) shown overlaid on a glass brain in three orthogonal planes and (B) shown overlaid on a coronal section (y = 3) of a structural template brain. (C) Graphical representations of the results in the left and right caudate are also shown. The plots demonstrate the fitted relationship between the effect sizes (parameter estimates) and the normalized reaction times to subsequent task-related stimuli for the novel sounds (red) and the deviant tones (blue). The effect size is expressed as percentage of the global mean intensity of the scans. The reaction times were normalized using a Euclidean Normalization method as implemented by SPM2. The dotted lines are 95% confidence intervals.
Fig. 3
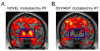
Brain activations to the (A) novel sounds and (B) deviant tones, each modulated by reaction times to subsequent task-related stimuli (RT), overlaid on coronal sections of a structural template brain ( y = 3). The significance threshold was leniently set to P < 0.50 uncorrected, confirming lack of striatal activity to the deviant tones modulated by RT (B). The striatal region is outlined by the blue rectangle.
Similar articles
- Human striatal responses to monetary reward depend on saliency.
Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Zink CF, et al. Neuron. 2004 May 13;42(3):509-17. doi: 10.1016/s0896-6273(04)00183-7. Neuron. 2004. PMID: 15134646 - Human striatal response to salient nonrewarding stimuli.
Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Zink CF, et al. J Neurosci. 2003 Sep 3;23(22):8092-7. doi: 10.1523/JNEUROSCI.23-22-08092.2003. J Neurosci. 2003. PMID: 12954871 Free PMC article. Clinical Trial. - Modulation of caudate activity by action contingency.
Tricomi EM, Delgado MR, Fiez JA. Tricomi EM, et al. Neuron. 2004 Jan 22;41(2):281-92. doi: 10.1016/s0896-6273(03)00848-1. Neuron. 2004. PMID: 14741108 - Differential cingulate and caudate activation following unexpected nonrewarding stimuli.
Davidson MC, Horvitz JC, Tottenham N, Fossella JA, Watts R, Uluğ AM, Casey BJ. Davidson MC, et al. Neuroimage. 2004 Nov;23(3):1039-45. doi: 10.1016/j.neuroimage.2004.07.049. Neuroimage. 2004. PMID: 15528104 Clinical Trial. - Emotional arousal amplifies the effects of biased competition in the brain.
Lee TH, Sakaki M, Cheng R, Velasco R, Mather M. Lee TH, et al. Soc Cogn Affect Neurosci. 2014 Dec;9(12):2067-77. doi: 10.1093/scan/nsu015. Epub 2014 Feb 14. Soc Cogn Affect Neurosci. 2014. PMID: 24532703 Free PMC article.
Cited by
- Distinct Roles of the Human Subthalamic Nucleus and Dorsal Pallidum in Parkinson's Disease Impulsivity.
Eisinger RS, Cagle JN, Alcantara JD, Opri E, Cernera S, Le A, Torres Ponce EM, Lanese J, Nelson B, Lopes J, Hundley C, Ravy T, Wu SS, Foote KD, Okun MS, Gunduz A. Eisinger RS, et al. Biol Psychiatry. 2022 Feb 15;91(4):370-379. doi: 10.1016/j.biopsych.2021.03.002. Epub 2021 Mar 6. Biol Psychiatry. 2022. PMID: 33993998 Free PMC article. - A biologically plausible embodied model of action discovery.
Bolado-Gomez R, Gurney K. Bolado-Gomez R, et al. Front Neurorobot. 2013 Mar 12;7:4. doi: 10.3389/fnbot.2013.00004. eCollection 2013. Front Neurorobot. 2013. PMID: 23487577 Free PMC article. - Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART).
Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Rao H, et al. Neuroimage. 2008 Aug 15;42(2):902-10. doi: 10.1016/j.neuroimage.2008.05.046. Epub 2008 Jun 3. Neuroimage. 2008. PMID: 18582578 Free PMC article. - Cracking the moody brain: the rewards of self starvation.
Zink CF, Weinberger DR. Zink CF, et al. Nat Med. 2010 Dec;16(12):1382-3. doi: 10.1038/nm1210-1382. Nat Med. 2010. PMID: 21135849 No abstract available. - A universal role of the ventral striatum in reward-based learning: evidence from human studies.
Daniel R, Pollmann S. Daniel R, et al. Neurobiol Learn Mem. 2014 Oct;114:90-100. doi: 10.1016/j.nlm.2014.05.002. Epub 2014 May 10. Neurobiol Learn Mem. 2014. PMID: 24825620 Free PMC article. Review.
References
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. - PubMed
- Boucsein W. Electrodermal Activity. Plenum Press; New York: 1992.
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DA016434/DA/NIDA NIH HHS/United States
- DA00367/DA/NIDA NIH HHS/United States
- DA016434/DA/NIDA NIH HHS/United States
- F31 MH067348/MH/NIMH NIH HHS/United States
- K08 DA000367/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources