Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae - PubMed (original) (raw)
Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae
Kyle I Happel et al. J Exp Med. 2005.
Abstract
Interleukin (IL)-23 is a heterodimeric cytokine that shares the identical p40 subunit as IL-12 but exhibits a unique p19 subunit similar to IL-12 p35. IL-12/23 p40, interferon gamma (IFN-gamma), and IL-17 are critical for host defense against Klebsiella pneumoniae. In vitro, K. pneumoniae-pulsed dendritic cell culture supernatants elicit T cell IL-17 production in a IL-23-dependent manner. However, the importance of IL-23 during in vivo pulmonary challenge is unknown. We show that IL-12/23 p40-deficient mice are exquisitely sensitive to intrapulmonary K. pneumoniae inoculation and that IL-23 p19-/-, IL-17R-/-, and IL-12 p35-/- mice also show increased susceptibility to infection. p40-/- mice fail to generate pulmonary IFN-gamma, IL-17, or IL-17F responses to infection, whereas p35-/- mice show normal IL-17 and IL-17F induction but reduced IFN-gamma. Lung IL-17 and IL-17F production in p19-/- mice was dramatically reduced, and this strain showed substantial mortality from a sublethal dose of bacteria (10(3) CFU), despite normal IFN-gamma induction. Administration of IL-17 restored bacterial control in p19-/- mice and to a lesser degree in p40-/- mice, suggesting an additional host defense requirement for IFN-gamma in this strain. Together, these data demonstrate independent requirements for IL-12 and IL-23 in pulmonary host defense against K. pneumoniae, the former of which is required for IFN-gamma expression and the latter of which is required for IL-17 production.
Figures
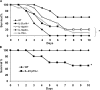
Figure 1.
Animals with targeted gene deletion in the IL-12, IL-17, and IL-23 signaling pathways demonstrate increased mortality during intrapulmonary K. pneumoniae infection. (A) WT C57BL/6, IL-23 p19−/−, IL-12 p35−/−, IL-12 p40−/−, or IL-17R−/− mice were challenged with 104 CFU intratracheal K. pneumoniae and survival was recorded every 12 h (n = 16–20 per group). IL-12 p40 knockout mice showed the greatest susceptibility to infection (*P < 0.01 compared with C57BL/6 [log rank test]). Survival differences between IL-23 p19−/−, IL-17R−/−, and IL-12 p35−/− mice were not statistically significant. (B) WT and p19−/− mice were also challenged with a lower dose (103 CFU) of bacteria, demonstrating significant mortality in p19−/− mice to a sublethal dose of K. pnuemoniae (*P < 0.01 compared with WT mice; n = 10 per group).
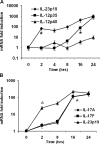
Figure 2.
Cytokine mRNA expression following pulmonary K. pneumoniae infection. Animals were administered 104 CFU bacteria and killed at specified time points. BAL cell pellet and lung homogenate mRNA were assayed via real-time RT-PCR. (A) Increases in BAL cell IL-23 p19, IL-12/23 p40, and IL-12 p35 mRNA expression during infection (n = 4–5 per group). (B) Whole lung tissue IL-23 p19, IL-17, and IL-17F mRNA expression following infection (n = 4–5 per group). Data are normalized for 18s ribosomal RNA content and plotted as fold change over baseline (time 0) expression. *Earliest significant (P < 0.05) increase in expression compared with time zero transcripts. Error bars represent mean ± SD.
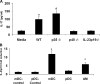
Figure 3.
AM IL-23 expression is required for induction of splenocyte IL-17 expression in response to K. pneumoniae. (A) AMs from naive WT, p35−/−, p40−/−, and p19−/− mice were recovered via BAL and exposed in vitro to K. pneumoniae. After 24 h, supernatants were harvested, centrifuged, and placed onto adherent cell-depleted WT splenocytes for 24 h to assay IL-17 induction (n = 5 per group; *P < 0.05 compared with media control). (B) IL-23 p19 mRNA expression in unexposed mDCs (mDC-control) or pDCs (pDC-control) or mDCs, AMs, and pDCs following 2-h in vitro exposure to K. pneumoniae. Data are expressed as fold increase in p19 expression compared with mDC- or pDC-control (n = 4–5 per group; *P < 0.05 compared with mDC-control). Error bars represent mean ± SD.
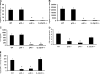
Figure 4.
IL-23 expression is required for lung IL-17 and IL-17F expression, whereas IL-12 is necessary for IFN-γ induction in response to K. pneumoniae infection. WT, p35−/−, p40−/−, and p19−/− mice were infected with 104 CFU K. pneumoniae and killed 24 h after infection. (A, B) Whole lung homogenate IL-17 (A) and IL-17F (B) mRNA expression as measured via real-time RT-PCR. (C) Whole lung homogenate IL-17 protein expression in repeat experiments. (D) Lung homogenate IFN-γ content in response to infection, indicating IL-23 is not sufficient to induce IFN-γ in the absence of IL-12. (E) Lung homogenate IFN-γ mRNA 24 h after K. pneumoniae infection confirms equivalent IFN-γ expression in WT and IL-23 p19−/− mice (n = 6 per group; *P < 0.05 compared with WT). Error bars represent mean ± SD.
Figure 5.
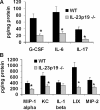
IL-23 p19−/− mice have reduced cytokine and chemokine responses to K. pneumoniae infection. Whole lung cytokine and chemokine content were measured in lung homogenates 24 h after 104 CFU K. pneumoniae delivery. Values are normalized to homogenate protein concentration (n = 6 per group; *P < 0.05 compared with WT). Error bars represent mean ± SD.
Figure 6.
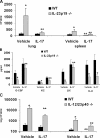
Defects in pulmonary host defense in IL-23 p19−/− mice are rescued by IL-17 treatment. WT C57BL/6 or IL-23 p19−/− mice were challenged with 104 K. pneumoniae followed by intratracheal administration of 1.5 μg recombinant murine IL-17 (or phosphate-buffered saline vehicle) 12 h later. Animals were then killed 24 h after rmIL-17 (or vehicle) delivery. (A) IL-17 improves bacterial clearance in IL-23 p19−/− mice. (B) IL-17 restores pulmonary concentrations of G-CSF, KC, and LIX in IL-23 p19−/− mice. (C) IL-17 partially improves bacterial clearance in IL-12 p40−/− mice. *P < 0.05 compared with WT vehicle-treated control. **P < 0.05 compared with vehicle-treated group of same genotype (n = 4–6 per group). Error bars represent mean ± SD.
Similar articles
- IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available.
Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. Khader SA, et al. J Immunol. 2005 Jul 15;175(2):788-95. doi: 10.4049/jimmunol.175.2.788. J Immunol. 2005. PMID: 16002675 - Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection.
Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Happel KI, et al. J Immunol. 2003 May 1;170(9):4432-6. doi: 10.4049/jimmunol.170.9.4432. J Immunol. 2003. PMID: 12707317 Free PMC article. - Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present.
Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Cooper AM, et al. J Immunol. 2002 Feb 1;168(3):1322-7. doi: 10.4049/jimmunol.168.3.1322. J Immunol. 2002. PMID: 11801672 - The role of IL-12, IL-23 and IFN-gamma in immunity to viruses.
Novelli F, Casanova JL. Novelli F, et al. Cytokine Growth Factor Rev. 2004 Oct;15(5):367-77. doi: 10.1016/j.cytogfr.2004.03.009. Cytokine Growth Factor Rev. 2004. PMID: 15450252 Free PMC article. Review. - IL-23 and IL-27: new members of the growing family of IL-12-related cytokines with important implications for therapeutics.
Cordoba-Rodriguez R, Frucht DM. Cordoba-Rodriguez R, et al. Expert Opin Biol Ther. 2003 Aug;3(5):715-23. doi: 10.1517/14712598.3.5.715. Expert Opin Biol Ther. 2003. PMID: 12880372 Review.
Cited by
- Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice.
Lee B, Robinson KM, McHugh KJ, Scheller EV, Mandalapu S, Chen C, Di YP, Clay ME, Enelow RI, Dubin PJ, Alcorn JF. Lee B, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Jul 15;309(2):L158-67. doi: 10.1152/ajplung.00338.2014. Epub 2015 May 22. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26001778 Free PMC article. - Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia.
Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, McDonald RA, White ES, Toews GB, Huffnagle GB. Murdock BJ, et al. Infect Immun. 2012 Apr;80(4):1424-36. doi: 10.1128/IAI.05529-11. Epub 2012 Jan 17. Infect Immun. 2012. PMID: 22252873 Free PMC article. - STAT1 Employs Myeloid Cell-Extrinsic Mechanisms to Regulate the Neutrophil Response and Provide Protection against Invasive Klebsiella pneumoniae Lung Infection.
Gonzalez-Ferrer S, Peñaloza HF, van der Geest R, Xiong Z, Gheware A, Tabary M, Kochin M, Dalton K, Zou H, Lou D, Lockwood K, Zhang Y, Bain WG, Mallampalli RK, Ray A, Ray P, Van Tyne D, Chen K, Lee JS. Gonzalez-Ferrer S, et al. Immunohorizons. 2024 Jan 1;8(1):122-135. doi: 10.4049/immunohorizons.2300104. Immunohorizons. 2024. PMID: 38289252 Free PMC article. - Phenotypic and functional features of human Th17 cells.
Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Annunziato F, et al. J Exp Med. 2007 Aug 6;204(8):1849-61. doi: 10.1084/jem.20070663. Epub 2007 Jul 16. J Exp Med. 2007. PMID: 17635957 Free PMC article. - Interleukin-1 receptor signaling is required to overcome the effects of pertussis toxin and for efficient infection- or vaccination-induced immunity against Bordetella pertussis.
Zhang X, Hester SE, Kennett MJ, Karanikas AT, Bendor L, Place DE, Harvill ET. Zhang X, et al. Infect Immun. 2011 Jan;79(1):527-41. doi: 10.1128/IAI.00590-10. Epub 2010 Oct 25. Infect Immun. 2011. PMID: 20974829 Free PMC article.
References
- Mehrad, B., and T.J. Standiford. 1999. Role of cytokines in pulmonary antimicrobial host defense. Immunol. Res. 20:15–27. - PubMed
- Zhang, P., W.R. Summer, G.J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39–51. - PubMed
- Ulich, T.R., L.R. Watson, S.M. Yin, K.Z. Guo, P. Wang, H. Thang, and J. del Castillo. 1991. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am. J. Pathol. 138:1485–1496. - PMC - PubMed
- Becker, S., R.B. Devlin, and J.S. Haskill. 1989. Differential production of tumor necrosis factor, macrophage colony stimulating factor, and interleukin 1 by human alveolar macrophages. J. Leukoc. Biol. 45:353–361. - PubMed
- Isler, P., B.G. de Rochemonteix, F. Songeon, N. Boehringer, and L.P. Nicod. 1999. Interleukin-12 production by human alveolar macrophages is controlled by the autocrine production of interleukin-10. Am. J. Respir. Cell Mol. Biol. 20:270–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08AA015163/AA/NIAAA NIH HHS/United States
- R37 HL079142/HL/NHLBI NIH HHS/United States
- P60 AA009803/AA/NIAAA NIH HHS/United States
- T32AA07577/AA/NIAAA NIH HHS/United States
- T32 AA007577/AA/NIAAA NIH HHS/United States
- R01HL079142/HL/NHLBI NIH HHS/United States
- R01HL061271/HL/NHLBI NIH HHS/United States
- P60AA009803/AA/NIAAA NIH HHS/United States
- R01AI051677/AI/NIAID NIH HHS/United States
- R01 AI051677/AI/NIAID NIH HHS/United States
- K08 AA015163/AA/NIAAA NIH HHS/United States
- R01 HL079142/HL/NHLBI NIH HHS/United States
- R01 HL061271/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous