Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder - PubMed (original) (raw)
Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder
Leonardo Salmena et al. J Exp Med. 2005.
Abstract
Caspase-8 is best known for its cell death function via death receptors. Recent evidence indicates that caspase-8 also has nonapoptotic functions. Caspase-8 deficiency is associated with pathologies that are unexpected for a proapoptotic molecule, such as abrogation of activation-induced lymphocyte proliferation, perturbed immune homeostasis, and immunodeficiency. In this study, we report the long-term physiological consequences of T cell-specific deletion of caspase-8 (tcasp8-/-). We show that tcasp8-/- mice develop an age-dependent lethal lymphoproliferative and lymphoinfiltrative immune disorder characterized by lymphoadenopathy, splenomegaly, and accumulation of T cell infiltrates in the lungs, liver, and kidneys. Peripheral casp8-/- T cells manifest activation marker up-regulation and are proliferating in the absence of any infection or stimulation. We also provide evidence suggesting that this immune disorder is different from the autoimmune lymphoproliferative syndrome. Interestingly, the condition described in tcasp8-/- mice manifests features consistent with the disorder described in humans with Caspase-8 deficiency. These findings suggest that tcasp8-/- mice may serve as an animal model to evaluate Caspase-8-deficient patient prognosis and therapy. Overall, our study uncovers novel in vivo functions for caspase-8 in immune regulation.
Figures
Figure 1.
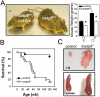
Decreased weight and decreased viability in old tcasp8 −/− mice. (A) Representative mice demonstrate relative sizes of old casp8 fl/fl;LckCre (Otcasp8 −/−) mice compared with casp8 fl/fl control mice. Old tcasp8−/− (n male = 5, n female = 14) weighed significantly less than their casp8fl /fl control littermates (n male = 5, n female = 10). All mice were ∼24 wk old. Error bars represent the mean ± SEM as described in Materials and methods. *, P ≤ 0.05. (B) Kaplan-Meier analysis represents the percent survival of control (n = 18) and tcasp8 − / − (n = 15) cohort mice versus age in weeks. (C) Representative LN and spleens demonstrate lymphoadenopathy and splenomegaly in old tcasp8 − / − mice. An asterisk indicates statistical significance.
Figure 2.
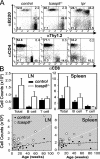
Lymphoproliferation in old tcasp8 −/− mice. (A) Representative flow cytometric analysis identified the proportion of T cells (Thy1.2+) versus B cells (B220+) and the proportion of CD4+ (Thy1.2+CD4+) versus CD8+ (Thy1.2+CD8+) T cells in control, tcasp8 − / −, and lpr mice. Numbers represent percentage of cells per quandrant. (B) Total lymphocyte, total B cell, and total T cell counts were evaluated in the LNs (top left) and spleen (top right). Total lymphocyte counts in the LNs and spleen were plotted against mouse age (bottom left and right, respectively). A line of best-fit was plotted on each graph. Error bars represent the mean ± SEM as described in Materials and methods.
Figure 3.
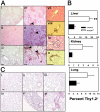
Age-dependent multitissue T cell infiltration in old tcasp8 −/− mice. (A) Liver (i, iv), kidney (ii, v), and lung (iii, vi) sections from 30-wk-old mice were immunohistochemically stained with anti-B220 antibodies (to reveal B cells) and anti-CD3 antibodies (to reveal T cells). Consecutive liver and kidney sections stained with hematoxylin-eosin (vii, ix) and anti-CD3 (viii, x) demonstrate focal interstitial T cell accumulation in perivascular (vii, viii) or periglomerular (ix, x) areas, respectively. (B) Single cell suspensions were prepared from the liver, kidneys, and lungs, and T cell infiltrates were quantitated via flow cytometry. This analysis was repeated in a minimum of three mice per genotype. Error bars represent the mean ± SEM as described in Materials and methods. **, P = 0.00486. (C) Analysis of lung tissue from 6-wk-old (i, iv), 20-wk-old (ii, v), and 50-wk-old (iii, vi) control (i–iii) and tcasp8 − / − (iv–vi) mice with anti-CD3 antibodies demonstrates an age-dependent increase in T cell infiltration. Lung tissues from control mice appear relatively normal at all ages (i–iii), whereas abnormal tissue is observed in the lung sections of 50-wk-old tcasp8 − / − mice (vi). Black arrowheads indicate areas of focal T cell accumulation. Bars indicate 0.1 mm.
Figure 4.
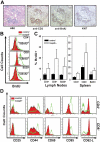
Infiltrating and circulating T cells from old tcasp8 −/− mice are proliferating and activated. In vivo BrdU incorporation was determined after injecting BrdU into mice. (A) Pulmonary T cell infiltrates in old tcasp8 − / − mice contained actively proliferating T cells as determined immunohistochemically via BrdU staining and Ki67 labeling. (B) Examination of circulating T cells via flow cytometry revealed a greater proportion of BrdU+ cells in each of the CD4+, CD8+, and B220+ lymphocyte compartments of old tcasp8 − / − mice. (C) BrdU incorporation in CD4+, CD8+, and B220+ lymphocytes was quantitated (n = 3) for each genotype. Error bars represent the mean ± SEM as described in Materials and methods. (D) Representative flow cytometry analysis of activation markers in peripheral T cells isolated from lymphoid organs isolated from old tcasp8 − / − mice and littermate controls. Cell surface expression of the activation markers CD69, CD44, CD25, and CD95 and the T cell memory marker CD62L on both CD4+ and CD8+ subsets of T cells are shown.
Figure 5.
Old tcasp8 −/− mice do not manifest systemic lupus erythematosus-like autoimmune phenotypes. (A) Levels of circulating IgG clonotypes and (B) anti-dsDNA antibodies were analyzed via ELISA in tail blood samples. Horizontal bars represent the mean. (C) Representative glomeruli from 30-wk-old control and tcasp8 − / − mice showing no accumulation of immune complexes compared with lpr mice manifesting high levels of glomerular immune complex accumulation.
Similar articles
- Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency.
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ. Chun HJ, et al. Nature. 2002 Sep 26;419(6905):395-9. doi: 10.1038/nature01063. Nature. 2002. PMID: 12353035 - Active immune response protects Stat6VT transgenic mice from developing a lymphoproliferative disorder.
Crane ED, Stephenson N, Haffner C, Bruns HA. Crane ED, et al. Immunobiology. 2010 Jul;215(7):579-85. doi: 10.1016/j.imbio.2009.09.001. Epub 2009 Oct 12. Immunobiology. 2010. PMID: 19822376 - Age-dependent, polyclonal hyperactivation of T cells is reduced in TNF-negative gld/gld mice.
Wiede F, Roomberg A, Cretney E, Lechner A, Fromm P, Wren L, Smyth MJ, Körner H. Wiede F, et al. J Leukoc Biol. 2009 Jan;85(1):108-16. doi: 10.1189/jlb.0107018. Epub 2008 Oct 23. J Leukoc Biol. 2009. PMID: 18948547 - Caspases signal not only apoptosis but also antigen-induced activation in cells of the immune system.
Newton K, Strasser A. Newton K, et al. Genes Dev. 2003 Apr 1;17(7):819-25. doi: 10.1101/gad.1077403. Genes Dev. 2003. PMID: 12670865 Review. No abstract available. - Vital functions for lethal caspases.
Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Launay S, et al. Oncogene. 2005 Aug 4;24(33):5137-48. doi: 10.1038/sj.onc.1208524. Oncogene. 2005. PMID: 16079910 Review.
Cited by
- Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis.
Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A. Osborn SL, et al. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13034-9. doi: 10.1073/pnas.1005997107. Epub 2010 Jul 6. Proc Natl Acad Sci U S A. 2010. PMID: 20615958 Free PMC article. - Receptor Interacting Protein Kinase Pathways Regulate Innate B Cell Developmental Checkpoints But Not Effector Function in Mice.
Parthasarathy R, Hägglöf T, Hadley JT, McLennan A, Mattke A, Dudley EA, Kumagai A, Dong LQ, Leadbetter EA. Parthasarathy R, et al. Front Immunol. 2021 Dec 9;12:758407. doi: 10.3389/fimmu.2021.758407. eCollection 2021. Front Immunol. 2021. PMID: 34956189 Free PMC article. - Programmed necrosis: backup to and competitor with apoptosis in the immune system.
Han J, Zhong CQ, Zhang DW. Han J, et al. Nat Immunol. 2011 Nov 16;12(12):1143-9. doi: 10.1038/ni.2159. Nat Immunol. 2011. PMID: 22089220 Review. - The regulation and role of c-FLIP in human Th cell differentiation.
Kyläniemi MK, Kaukonen R, Myllyviita J, Rasool O, Lahesmaa R. Kyläniemi MK, et al. PLoS One. 2014 Jul 14;9(7):e102022. doi: 10.1371/journal.pone.0102022. eCollection 2014. PLoS One. 2014. PMID: 25019384 Free PMC article. - Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia.
Kim SJ, Li J. Kim SJ, et al. Cell Death Dis. 2013 Jul 11;4(7):e716. doi: 10.1038/cddis.2013.238. Cell Death Dis. 2013. PMID: 23846218 Free PMC article.
References
- Green, D.R., N. Droin, and M. Pinkoski. 2003. Activation-induced cell death in T cells. Immunol. Rev. 193:70–81. - PubMed
- Boldin, M.P., T.M. Goncharov, Y.V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 85:803–815. - PubMed
- Muzio, M., A.M. Chinnaiyan, F.C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J.D. Bretz, M. Zhang, R. Gentz, et al. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell. 85:817–827. - PubMed
- Su, H., N. Bidere, L. Zheng, A. Cubre, K. Sakai, J. Dale, L. Salmena, R. Hakem, S. Straus, and M. Lenardo. 2005. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 307:1465–1468. - PubMed
- Chun, H.J., L. Zheng, M. Ahmad, J. Wang, C.K. Speirs, R.M. Siegel, J.K. Dale, J. Puck, J. Davis, C.G. Hall, et al. 2002. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 419:395–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous