Dynamic cycling of eIF2 through a large eIF2B-containing cytoplasmic body: implications for translation control - PubMed (original) (raw)
Comparative Study
Dynamic cycling of eIF2 through a large eIF2B-containing cytoplasmic body: implications for translation control
Susan G Campbell et al. J Cell Biol. 2005.
Abstract
The eukaryotic translation initiation factor 2B (eIF2B) provides a fundamental controlled point in the pathway of protein synthesis. eIF2B is the heteropentameric guanine nucleotide exchange factor that converts eIF2, from an inactive guanosine diphosphate-bound complex to eIF2-guanosine triphosphate. This reaction is controlled in response to a variety of cellular stresses to allow the rapid reprogramming of cellular gene expression. Here we demonstrate that in contrast to other translation initiation factors, eIF2B and eIF2 colocalize to a specific cytoplasmic locus. The dynamic nature of this locus is revealed through fluorescence recovery after photobleaching analysis. Indeed eIF2 shuttles into these foci whereas eIF2B remains largely resident. Three different strategies to decrease the guanine nucleotide exchange function of eIF2B all inhibit eIF2 shuttling into the foci. These results implicate a defined cytoplasmic center of eIF2B in the exchange of guanine nucleotides on the eIF2 translation initiation factor. A focused core of eIF2B guanine nucleotide exchange might allow either greater activity or control of this elementary conserved step in the translation pathway.
Figures
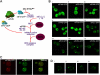
Figure 1.
Localization of eIFs in S. cerevisiae . (A) Diagram representing the eukaryotic translation initiation pathway. (B) Live cell confocal microscopic images of strains YMK1170, 1171, 1172, 885, 881, 883, 1211, 880, and 882 bearing chromosomally integrated COOH-terminal eGFP tags, (i) TIF1-GFP (eIF4AI-GFP), (ii) TIF5-GFP (eIF5-GFP), (iii) TIF4631-GFP (eIF4GI-GFP), (iv) CDC33-GFP (eIF4E-GFP), (v) PRT1-GFP (eIF3b-GFP), (vi) SUI2-GFP (eIF2α-GFP), (vii) GCD11-GFP (eIF2γ-GFP), (viii) GCD1-GFP (eIF2Bγ-GFP), and (ix) GCD6-GFP (eIF2Bɛ-GFP). (C) Colocalization (left) GCD1-CFP (eIF2Bγ-GFP), (middle) SUI2-YFP (eIF2α-GFP), and (right, overlay) using strain YMK1144. (D) Immunofluorescence of fixed YMK467 cells with anti-eIF2Bɛ antibodies. Four defined images from the same field of view are shown.
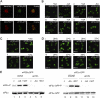
Figure 2.
The eIF2–eIF2B foci are not sites of eIF2B regulation or TC formation however they do require active translation. (A) The localization of (i) initiator and (ii) elongator Met-tRNAMet was analyzed in the eIF2Bγ-GFP–containing strain YMK880 by FISH using end-labeled oligonucleotides. FISH images were compared with eIF2Bγ-GFP localization in the overlay images. (B) GCN2 and _gcn2_-null strains bearing eIF2Bγ-GFP, YMK880 (i) and YMK1087 (ii), and eIF2α-GFP, YMK883 (iii), and YMK1088 (iv) were grown in media containing (+AA) or lacking amino acids (−AA) for 15 min. Cells were visualized by live cell confocal microscopy. (C) Strains YMK880 (i, eIF2Bγ-GFP) and YMK883 (ii, eIF2α-GFP) were incubated at room temperature for 10 min in the presence or absence of cycloheximide (100 μg/ml). (D) The strains (i) YMK1123 (eIF2Bγ-GFP, prt1-1), (ii) YMK880 (eIF2Bγ-GFP), (iii) YMK1124 (eIF2α-GFP, prt1-1), and (iv) YMK883 (eIF2α-GFP) were incubated at the permissive (26°C) and nonpermissive (37°C) temperature for 15 min. Cells were visualized by live cell confocal microscopy. (E) Protein extracts from eIF2Bγ-GFP strains YMK880 (GCN2, lanes 1–3), YMK1087 (_gcn2_Δ, lanes 4–6), and eIF2α-GFP strains, YMK883 (GCN2, lanes 8–10), and YMK1088 (_gcn2_Δ, lanes 11–13) were blotted and probed with antibodies to eIF2α and phosphospecific antibodies to phophoserine 51 on eIF2α.
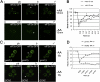
Figure 3.
eIF2 cycles rapidly through the foci whereas eIF2B is less dynamic. (A) eIF2α-GFP FRAP analysis. Panels show representative prebleach (pb), bleach (b), and recovery (r) images from FRAP experiment on strain YMK883. The bleached focus is marked with a white arrowhead and the asterisk on the recovery image corresponds to time point on the graph when the image was taken. (B) Graph show quantitation of eIF2α-GFP FRAP experiments. Control represents the FRAP results from YMK883 fixed cells. (C) eIF2Bγ-GFP FRAP analysis. Panels show representative prebleach (pb), bleach (b), and recovery (r) images from FRAP experiment on strain YMK880. The bleached focus is marked with a white arrowhead and the asterisk on the recovery image corresponds to time point on the graph when the image was taken. (D) Graph show quantitation of eIF2Bγ-GFP FRAP experiments. Control represents the FRAP results from YMK880-fixed cells.
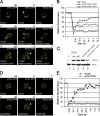
Figure 4.
eIF2α-GFP shuttling is altered in the absence of amino acids. Figure shows FRAP experiments on eIF2α-GFP–bearing strains as described in Fig. 3. (A) YMK883 FRAP after (i) 15-min control incubation and (ii) 15-min starvation for amino acids. (B) Graph showing quantitation of eIF2α-GFP amino acid starvation FRAP experiments. (C) YMK1088 (i, _gcn2_Δ) and (ii) YMK883 strains after 1 h starvation for amino acids. (D) Graph showing quantitation of eIF2α-GFP FRAP experiments after a 1-h amino acid starvation in the presence and absence of Gcn2p. pb, Prebleach; b, bleach; and r, recovery.
Figure 5.
The eIF2–eIF2B foci represent sites of guanine nucleotide exchange. Figure shows FRAP experiments on eIF2α-GFP bearing strains as described in Fig. 3. (A) YMK883 strains transformed with (i) control plasmid, pRS316, (ii) pAV1245 (GCN2 cM788V-E1606G), and (iii) pAV1248 (GCN2 cM788-E1591K), respectively. (B) Graph showing quantitation of eIF2α-GFP FRAP experiments with GCN2 c mutants. (C) Protein extracts from strains YMK883 pRS316, YMK883 pAV1245[GCN2 cM788V-E1606G], and YMK883 pAV1248[GCN2 cM788V-E1591K] were blotted and probed with antibodies to eIF2α and phosphospecific antibodies to phophoserine 51 on eIF2α. (D) FRAP analysis of eIF2α-GFP in strains (i) YMK1168 (GCD6) and (ii) YMK1169 (gcd6-F250L). (E) Graph showing quantitation of eIF2α-GFP FRAP experiments with wt and eIF2Bɛ catalytic mutant. pb, Prebleach; b, bleach; r, recovery.
Similar articles
- The beta/Gcd7 subunit of eukaryotic translation initiation factor 2B (eIF2B), a guanine nucleotide exchange factor, is crucial for binding eIF2 in vivo.
Dev K, Qiu H, Dong J, Zhang F, Barthlme D, Hinnebusch AG. Dev K, et al. Mol Cell Biol. 2010 Nov;30(21):5218-33. doi: 10.1128/MCB.00265-10. Epub 2010 Aug 30. Mol Cell Biol. 2010. PMID: 20805354 Free PMC article. - An eIF5/eIF2 complex antagonizes guanine nucleotide exchange by eIF2B during translation initiation.
Singh CR, Lee B, Udagawa T, Mohammad-Qureshi SS, Yamamoto Y, Pavitt GD, Asano K. Singh CR, et al. EMBO J. 2006 Oct 4;25(19):4537-46. doi: 10.1038/sj.emboj.7601339. Epub 2006 Sep 21. EMBO J. 2006. PMID: 16990799 Free PMC article. - Localization of the translational guanine nucleotide exchange factor eIF2B: a common theme for GEFs?
Campbell SG, Ashe MP. Campbell SG, et al. Cell Cycle. 2006 Apr;5(7):678-80. doi: 10.4161/cc.5.7.2607. Epub 2006 Apr 1. Cell Cycle. 2006. PMID: 16582624 Review. - Protection of eIF2B from inhibitory phosphorylated eIF2: A viral strategy to maintain mRNA translation during the PKR-triggered integrated stress response.
Ito T, Wuerth JD, Weber F. Ito T, et al. J Biol Chem. 2023 Nov;299(11):105287. doi: 10.1016/j.jbc.2023.105287. Epub 2023 Sep 22. J Biol Chem. 2023. PMID: 37742919 Free PMC article. Review.
Cited by
- Relocalization of Translation Termination and Ribosome Recycling Factors to Stress Granules Coincides with Elevated Stop-Codon Readthrough and Reinitiation Rates upon Oxidative Stress.
Makeeva DS, Riggs CL, Burakov AV, Ivanov PA, Kushchenko AS, Bykov DA, Popenko VI, Prassolov VS, Ivanov PV, Dmitriev SE. Makeeva DS, et al. Cells. 2023 Jan 8;12(2):259. doi: 10.3390/cells12020259. Cells. 2023. PMID: 36672194 Free PMC article. - Regulation and function of elF2B in neurological and metabolic disorders.
Hanson FM, Hodgson RE, de Oliveira MIR, Allen KE, Campbell SG. Hanson FM, et al. Biosci Rep. 2022 Jun 30;42(6):BSR20211699. doi: 10.1042/BSR20211699. Biosci Rep. 2022. PMID: 35579296 Free PMC article. Review. - Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae.
Dever TE, Kinzy TG, Pavitt GD. Dever TE, et al. Genetics. 2016 May;203(1):65-107. doi: 10.1534/genetics.115.186221. Genetics. 2016. PMID: 27183566 Free PMC article. Review. - Filamentation of Metabolic Enzymes in Saccharomyces cerevisiae.
Shen QJ, Kassim H, Huang Y, Li H, Zhang J, Li G, Wang PY, Yan J, Ye F, Liu JL. Shen QJ, et al. J Genet Genomics. 2016 Jun 20;43(6):393-404. doi: 10.1016/j.jgg.2016.03.008. Epub 2016 Apr 1. J Genet Genomics. 2016. PMID: 27312010 Free PMC article. - Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation.
Ron D, Harding HP. Ron D, et al. Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a013177. doi: 10.1101/cshperspect.a013177. Cold Spring Harb Perspect Biol. 2012. PMID: 23209157 Free PMC article. Review.
References
- Anderson, P., and N. Kedersha. 2002. Stressful Initiations. J. Cell Sci. 115:3227–3234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases