Analysis of putative Chlamydia trachomatis chaperones Scc2 and Scc3 and their use in the identification of type III secretion substrates - PubMed (original) (raw)
Analysis of putative Chlamydia trachomatis chaperones Scc2 and Scc3 and their use in the identification of type III secretion substrates
Kenneth A Fields et al. J Bacteriol. 2005 Sep.
Abstract
The obligate intracellular pathogen Chlamydia trachomatis expresses a type III secretion system (T3SS) which has the potential to contribute significantly to pathogenesis. Based on a demonstrated role of type III secretion (T3S)-specific chaperones in the secretion of antihost proteins by gram-negative pathogens, we initiated a study of selected putative Chlamydia T3S chaperones in an effort to gain mechanistic insight into the Chlamydia T3SS and to potentially identify Chlamydia-specific secreted products. C. trachomatis Scc2 and Scc3 are homologous to SycD of Yersinia spp. Functional studies of the heterologous Yersinia T3SS indicated that although neither Scc2 nor Scc3 was able to fully complement a sycD null mutant, both have SycD-like characteristics. Both were able to associate with the translocator protein YopD, and Scc3 expression restored limited secretion of YopD in in vitro studies of T3S. CopB (CT578) and CopB2 (CT861) are encoded adjacent to scc2 and scc3, respectively, and have structural similarities with the YopB family of T3S translocators. Either Scc2 or Scc3 coprecipitates with CopB from C. trachomatis extracts. Expression of CopB or CopB2 in Yersinia resulted in their type III-dependent secretion, and localization studies with C. trachomatis-infected cells indicated that both were secreted by Chlamydia.
Figures
FIG. 1.
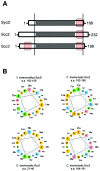
Comparison of C. trachomatis Scc2 and Scc3 with Yersinia SycD. (A) Schematic representations of proteins, indicating numbers of total residues. Vertical bars bracket domains in which sequence similarities are found. Respective TPR are shown as shaded areas, and amphipathic helices are represented by red boxes. (B) Amphipathic characters of predicted α helices are shown in helical wheel projections (
http://cti.itc.virginia.edu/∼cmg/Demo/wheel/wheelApp.html
), where nonpolar residues are shown in orange, polar, uncharged residues in green, acidic residues in pink, and basic residues in blue. Residue numbers of corresponding domains are provided for respective proteins. a.a., amino acids.
FIG. 2.
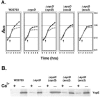
Complementation analysis of Y. enterocolitica sycD. The Y. enterocolitica wild type (W22703) and sycD (Δ_sycD_) expressing vector alone or SycD-FT, Scc2-FT, or Scc3-FT were cultivated with 0.1 mM IPTG and in the presence (⧫ [black lines] or +) or absence (• [grey lines] or −) of 2.5 mM Ca2+. Bacterial growth experiments were repeated four times, and a representative data set in which growth was plotted hourly by measuring optical density at _A_620 is shown (A). Cultures were harvested 4 h after temperature shift (indicated by arrows) to 37°C, and material corresponding to 0.05 OD620/ml of cell-free culture supernatants was resolved in 12% (wt/vol) polyacrylamide gels (B). The immunoblot was analyzed using α-YopE, and proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies, followed by development with NBT-BCIP.
FIG. 3.
Complementation of YopD secretion. Y. enterocolitica sycD expressing SycD-FT, Scc1-FT, Scc2-FT, and Scc3-FT or Y. enterocolitica WT and sycD (Δ_sycD_) were cultivated in the presence (+) or absence (−) of 2.5 mM Ca2+. Cultures were incubated at 26°C for 2 h and then shifted to 37°C. IPTG was added to 0.1 mM at the time of temperature shift to achieve induction of trans genes. After 4 h of growth at 37°C, 1.0-ml volumes were harvested and directly precipitated with TCA for TC samples or fractionated into samples representing CS prior to concentration. Material corresponding to 0.05 OD620/ml of original cultures for CS and 0.02 OD620/ml for TC was resolved in 12% (wt/vol) polyacrylamide gels. Flag-tagged SycD, Scc1, Scc2, and Scc3 were detected by immunoblotting with α-Flag M2, and YopD and YopE were detected with α-YopD and α-YopE, respectively. Immunoblots were probed with β-lactamase-specific antibodies as a control for cell lysis. Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies and development with NBT-BCIP.
FIG. 4.
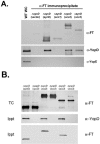
Scc2 and Scc3 coprecipitate with YopD. Cleared lysates of Y. enterocolitica sycD (Δ_sycD_) or yopD (Δ_yopD_) expressing vector or Flag-tagged proteins were generated from +Ca cultures (cultivated in the presence of 0.1 mM IPTG) harvested 4 h after temperature shift. (A) Flag-tagged proteins were immunoprecipitated from lysates with α-FlagM2 resin, and purified material was examined by immunoblotting with Flag-tag-specific antibodies (α-FT), α-YopD, or α-YopE. A lane containing a WC lysate from WT Y. enterocolitica was included as a positive control for α-YopD or α-YopE reactivity. (B) YopD was specifically immunoprecipitated by addition of α-YopD protein A-conjugated beads to lysates. Proteins in precipitate material (Ippt) were detected by immunoblotting with α-YopD or α-FT. Parallel TC lysates were also generated from cultures and probed by immunoblotting with α-FT. Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies, followed by development with NBT-BCIP.
FIG. 5.
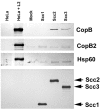
Scc2 and Scc3 coprecipitate CopB from _C. trachomatis_-containing lysates. Scc1-FT, Scc2-FT, Scc3-FT, and PBS as a mock-treated control (Mock) were incubated with cleared lysates from purified C. trachomatis RB and EB developmental forms. Flag-tagged proteins were purified by addition of α-FlagM2 resin, and material was analyzed by immunoblotting with CopB-, CopB2-, Hsp60-, or FT-specific antibodies. Lanes containing whole-culture lysates from mock-infected (HeLa) or _C. trachomatis_-infected (HeLa + L2) HeLa cell cultures were included as a control for antibody specificity. Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies, followed by development with NBT-BCIP.
FIG. 6.
CopB and CopB2 are T3S substrates. Y. pseudotuberculosis YPIII pIB102 (WT) expressing pCopBnpt, pCopB2NT, pScc2-FT, pScc3-FT, or YPIII pIB68 (yscS) expressing pCopBnpt or pCopB2NT were cultivated in HIB in the presence (+) or absence (−) of 2.5 mM Ca2+ (lanes 5 to 8). Strains expressing vector only as a negative control were included (lanes 1 to 4). After an initial 2-h incubation at 26°C, IPTG was added to 0.1 mM and cultures were shifted to 37°C. Cultures were harvested after 4 h of growth at 37°C and fractionated into samples representing CS and WC. Material corresponding to 0.10 OD620/ml of original cultures for CS and 0.02 OD620/ml for WC was resolved in 12% (wt/vol) polyacrylamide gels and analyzed by immunoblotting with α-Flag M2 (A and B) or α-SycD (A). For immunoblotting, proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies, followed by development with NBT-BCIP. C.t., C. trachomatis.
FIG. 7.
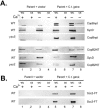
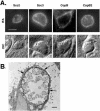
Immunolocalization of Scc2, Scc3, CopB, and CopB2 in _C. trachomatis_-infected HeLa monolayers. Proteins were detected 20 h postinfection via (A) indirect immunofluorescence or (B) immunoelectron microscopy. (A) Immunofluorescent (IFA) and Nomarski (DIC) images of individual inclusions are shown. Proteins were visualized by probing with Texas Red-conjugated secondary antibodies. Bar = 5 μm. (B) CopB was specifically stained by the immunoperoxidase method and examined by transmission electron microscopy. Arrows indicate the CopB-specific signal localized to the inclusion membrane. Bar = 1 μm.
FIG. 8.
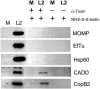
Accessibility of CopB2 to NHS-S-S-biotin in cells permeabilized with staphylococcal alpha-toxin. _C. trachomatis_-infected (L2) or mock-infected (M) HeLa cultures were treated with HBSS with (+) or without (−) alpha-toxin (α-Toxin), and susceptible proteins were labeled by addition of NHS-S-S-biotin. Material from whole-culture lysates (first lane M and first lane L2) or containing purified, biotinylated proteins from disrupted cultures was resolved in 12% (wt/vol) polyacrylamide gels and analyzed by immunoblotting using α-CopB2 or antibodies specific for bacterium-associated proteins MOMP, EfTu, and Hsp60 or extrainclusion-localized Chlamydia CADD. Proteins were visualized by probing with horseradish peroxidase-conjugated secondary antibodies, followed by chemiluminescent development with Super Signal substrate. All images are taken from equivalent exposures.
Similar articles
- Structure of the Yersinia enterocolitica type III secretion translocator chaperone SycD.
Büttner CR, Sorg I, Cornelis GR, Heinz DW, Niemann HH. Büttner CR, et al. J Mol Biol. 2008 Jan 25;375(4):997-1012. doi: 10.1016/j.jmb.2007.11.009. Epub 2007 Nov 12. J Mol Biol. 2008. PMID: 18054956 - Identification of novel type III secretion chaperone-substrate complexes of Chlamydia trachomatis.
Pais SV, Milho C, Almeida F, Mota LJ. Pais SV, et al. PLoS One. 2013;8(2):e56292. doi: 10.1371/journal.pone.0056292. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431368 Free PMC article. - Biochemical and localization analyses of putative type III secretion translocator proteins CopB and CopB2 of Chlamydia trachomatis reveal significant distinctions.
Chellas-Géry B, Wolf K, Tisoncik J, Hackstadt T, Fields KA. Chellas-Géry B, et al. Infect Immun. 2011 Aug;79(8):3036-45. doi: 10.1128/IAI.00159-11. Epub 2011 May 23. Infect Immun. 2011. PMID: 21606186 Free PMC article. - Conserved type III secretion system exerts important roles in Chlamydia trachomatis.
Dai W, Li Z. Dai W, et al. Int J Clin Exp Pathol. 2014 Aug 15;7(9):5404-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337183 Free PMC article. Review. - Type III Secretion in Chlamydia.
Rucks EA. Rucks EA. Microbiol Mol Biol Rev. 2023 Sep 26;87(3):e0003423. doi: 10.1128/mmbr.00034-23. Epub 2023 Jun 26. Microbiol Mol Biol Rev. 2023. PMID: 37358451 Free PMC article. Review.
Cited by
- Genus-optimized strategy for the identification of chlamydial type III secretion substrates.
Hovis KM, Mojica S, McDermott JE, Pedersen L, Simhi C, Rank RG, Myers GS, Ravel J, Hsia RC, Bavoil PM. Hovis KM, et al. Pathog Dis. 2013 Dec;69(3):213-22. doi: 10.1111/2049-632X.12070. Epub 2013 Aug 14. Pathog Dis. 2013. PMID: 23873765 Free PMC article. - Interactions between CdsD, CdsQ, and CdsL, three putative Chlamydophila pneumoniae type III secretion proteins.
Johnson DL, Stone CB, Mahony JB. Johnson DL, et al. J Bacteriol. 2008 Apr;190(8):2972-80. doi: 10.1128/JB.01997-07. Epub 2008 Feb 15. J Bacteriol. 2008. PMID: 18281400 Free PMC article. - SINC, a type III secreted protein of Chlamydia psittaci, targets the inner nuclear membrane of infected cells and uninfected neighbors.
Mojica SA, Hovis KM, Frieman MB, Tran B, Hsia RC, Ravel J, Jenkins-Houk C, Wilson KL, Bavoil PM. Mojica SA, et al. Mol Biol Cell. 2015 May 15;26(10):1918-34. doi: 10.1091/mbc.E14-11-1530. Epub 2015 Mar 18. Mol Biol Cell. 2015. PMID: 25788290 Free PMC article. - A working model for the type III secretion mechanism in Chlamydia.
Ferrell JC, Fields KA. Ferrell JC, et al. Microbes Infect. 2016 Feb;18(2):84-92. doi: 10.1016/j.micinf.2015.10.006. Epub 2015 Oct 26. Microbes Infect. 2016. PMID: 26515030 Free PMC article. Review. - Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle.
Wolf K, Betts HJ, Chellas-Géry B, Hower S, Linton CN, Fields KA. Wolf K, et al. Mol Microbiol. 2006 Sep;61(6):1543-55. doi: 10.1111/j.1365-2958.2006.05347.x. Mol Microbiol. 2006. PMID: 16968227 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous