Without nerves, immunology remains incomplete -in vivo veritas - PubMed (original) (raw)
Review
Without nerves, immunology remains incomplete -in vivo veritas
Andrew J Shepherd et al. Immunology. 2005 Oct.
Abstract
Interest in the interactions between nervous and immune systems involved in both pathological and homeostatic mechanisms of host defence has prompted studies of neuroendocrine immune modulation and cytokine involvement in neuropathologies. In this review we concentrate on a distinct area of homeostatic control of both normal and abnormal host defence activity involving the network of peripheral c-fibre nerve fibres. These nerve fibres have long been recognized by dermatologists and gastroenterologists as key players in abnormal inflammatory processes, such as dermatitis and eczema. However, the involvement of nerves can all too easily be regarded as that of isolated elements in a local phenomenon. On the contrary, it is becoming increasingly clear that neural monitoring of host defence activities takes place, and that involvement of central/spinal mechanisms are crucial in the co-ordination of the adaptive response to host challenge. We describe studies demonstrating neural control of host defence and use the specific examples of bone marrow haemopoiesis and contact sensitivity to highlight the role of direct nerve fibre connections in these activities. We propose a host monitoring system that requires interaction between specialized immune cells and nerve fibres distributed throughout the body and that gives rise to both neural and immune memories of prior challenge. While immunological mechanisms alone may be sufficient for local responsiveness to subsequent challenge, data are discussed that implicate the neural memory in co-ordination of host defence across the body, at distinct sites not served by the same nerve fibres, consistent with central nervous mediation.
Figures
Figure 1
Immunomodulation by the nervous system. Adrenocorticotrophic hormone (ACTH) released from the pituitary (centre) acts upon the adrenal cortex to stimulate the release of glucocorticoids (bottom left), which typically suppress immune function, especially biasing towards T helper 2 (Th2) cells. The adrenal medulla receives sympathetic innervation from the splanchnic nerves, which can provoke release of catecholamines into the circulation. Cytokine release and autoimmune processes within the central nervous system (top left) are also considered to be a form of neuroimmunomodulation. Sympathetic innervation (bottom right) is present in all lymphoid organs, as well as tertiary sites. Sensory innervation (centre right) is similarly pervasive and is characterized by peptide neurotransmitter release and capsaicin sensitivity. Parasympathetic innervation, (top right) principally from the vagus nerve, releases acetylcholine. Leucocytes have been shown to harbour receptors for and respond to all of these chemical mediators. CNS, central nervous system; IL-1, interleukin-1.
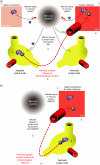
Figure 2
Effect of sensory denervation on the manifestation of contact sensitivity (CS). (a) Challenge at a site remote to sensitization: nerves are required to relay activation signals for CS between distant sites. Upon hapten sensitization of the abdomen (1), a signal is generated in the c-fibre associated with the Langerhans' cells (LC). The LC migrate to the draining lymph node (2) and interact with naïve, hapten-specific T lymphocytes. Upon ear challenge 4 days later (3), a second signal reaches the nervous system, prompting efferent signalling (4) to the inguinal lymph node (which harbours memory T cells from sensitization), as well as the vasculature and skin cells at the site of challenge. This stimulus evokes the release of memory T cells into the circulation (5), whereupon they migrate to the site of challenge and secrete a variety of pro-inflammatory mediators, contributing to contact sensitivity (6). When the site of sensitization is denervated with capsaicin 48 hr beforehand (top left asterisk), hapten sensitization of the abdomen does not generate a nervous system signal. However, LC migration to the local (inguinal) node and memory T-cell generation is unaffected. There is now a discrepancy in the system; the animal has immunological memory of exposure to the hapten, but no neural memory was formed. Upon challenge of an innervated site (ear), the nervous system receives the appropriate signal, but fails to mobilize memory T cells from the remote (abdominal–inguinal) lymph node. This, in turn, means that no recordable CS response occurs. Denervation of the site of challenge (top right asterisk) does not affect the propagation of a sensitization signal or formation of immunological memory, but a signal is not received from the site of challenge, meaning that no elicitation of the neural memory occurs, efferent signals are not generated and immunological memory is not mobilized, rendering the CS response absent. Denervation of the lymph node draining the site of sensitization (bottom asterisk) does not prevent the formation of neural or immunological memory, but the signal to the lymph node following challenge cannot be delivered. Consequently, mobilization of immunological memory fails and no CS response takes place. Loss of the CS response is reversed by the artificial infusion of substance P (SP) to the denervated node (data not shown). (b) Sensitization and challenge at the same site: local mechanisms exist for the activation of CS that are independent of nerves. Denervation of the site of sensitization or the draining lymph node does not preclude the formation of immunological memory. Following sensitization (1), a population of memory T cells, specific for hapten, are formed and remain in the draining lymph node (2). If the same site is used for challenge 4 days later, LC harbouring antigen will migrate to this same node, prompting effector T-cell release and migration to the site of challenge (3,4), initiating the response independently of nervous system input (5). The site of challenge and/or the draining lymph node can be denervated in this situation with no visible effect on the CS response. HEV, high endothelial venule; VE, vascular endothelia.
Similar articles
- The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease.
Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, von Andrian UH. Ordovas-Montanes J, et al. Trends Immunol. 2015 Oct;36(10):578-604. doi: 10.1016/j.it.2015.08.007. Trends Immunol. 2015. PMID: 26431937 Free PMC article. Review. - Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease.
Downing JE, Miyan JA. Downing JE, et al. Immunol Today. 2000 Jun;21(6):281-9. doi: 10.1016/s0167-5699(00)01635-2. Immunol Today. 2000. PMID: 10825740 Review. - Central neural activation following contact sensitivity peripheral immune challenge: evidence of brain-immune regulation through C fibres.
Thinschmidt JS, King MA, Korah M, Perez PD, Febo M, Miyan J, Grant MB. Thinschmidt JS, et al. Immunology. 2015 Oct;146(2):206-16. doi: 10.1111/imm.12479. Epub 2015 Aug 24. Immunology. 2015. PMID: 25967648 Free PMC article. - Neuroimmune communication in skin: far from peripheral.
Hendrix S. Hendrix S. J Invest Dermatol. 2008 Feb;128(2):260-1. doi: 10.1038/sj.jid.5701171. J Invest Dermatol. 2008. PMID: 18195741 Review. - Integrative neuroimmunomodulation of gastrointestinal function during enteric parasitism.
Palmer JM, Greenwood-Van Meerveld B. Palmer JM, et al. J Parasitol. 2001 Jun;87(3):483-504. doi: 10.1645/0022-3395(2001)087[0483:INOGFD]2.0.CO;2. J Parasitol. 2001. PMID: 11426710 Review.
Cited by
- The peripheral nervous system supports blood cell homing and survival in the Drosophila larva.
Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. Makhijani K, et al. Development. 2011 Dec;138(24):5379-91. doi: 10.1242/dev.067322. Epub 2011 Nov 9. Development. 2011. PMID: 22071105 Free PMC article. - Regional differences in cell-mediated immunity in people with diabetic peripheral neuropathy.
Pickwell K, Geerts M, van Moorsel D, Hilkman D, Kars M, Schaper NC. Pickwell K, et al. Diabet Med. 2020 Feb;37(2):350-355. doi: 10.1111/dme.14143. Epub 2019 Oct 10. Diabet Med. 2020. PMID: 31557355 Free PMC article. - Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds.
Buckley G, Wong J, Metcalfe AD, Ferguson MW. Buckley G, et al. J Anat. 2012 Jan;220(1):3-12. doi: 10.1111/j.1469-7580.2011.01452.x. Epub 2011 Nov 8. J Anat. 2012. PMID: 22066944 Free PMC article. - Drosophila as a model for the two myeloid blood cell systems in vertebrates.
Gold KS, Brückner K. Gold KS, et al. Exp Hematol. 2014 Aug;42(8):717-27. doi: 10.1016/j.exphem.2014.06.002. Epub 2014 Jun 17. Exp Hematol. 2014. PMID: 24946019 Free PMC article. - The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease.
Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, von Andrian UH. Ordovas-Montanes J, et al. Trends Immunol. 2015 Oct;36(10):578-604. doi: 10.1016/j.it.2015.08.007. Trends Immunol. 2015. PMID: 26431937 Free PMC article. Review.
References
- Lubahn CL, Schaller JA, Bellinger DL, Sweeney S, Lorton D. The importance of timing of adrenergic drug delivery in relation to the induction and onset of adjuvant-induced arthritis. Brain Behav Immun. 2004;18:563–71. - PubMed
- Harle P, Mobius D, Carr DJJ, Scholmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheumatism. 2005;52:1305–13. - PubMed
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. - PubMed
- Gibson RM, Rothwell NJ, Le Feuvre RA. CNS injury: the role of the cytokine IL-1. Vet J. 2004;168:230–7. - PubMed
- Berczi I. Neuroimmune biology – an introduction. In: Berczi I, Gorczynski RM, editors. New Foundation of Biology. Oxford: Elsevier Science; 2001. pp. 1–46.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources