Structural basis for the enhancement of eIF4A helicase activity by eIF4G - PubMed (original) (raw)
Structural basis for the enhancement of eIF4A helicase activity by eIF4G
Monika Oberer et al. Genes Dev. 2005.
Abstract
The eukaryotic translation initiation factors 4A (eIF4A) and 4G (eIF4G) are crucial for the assembly of the translationally active ribosome. Together with eIF4E, they form the eIF4F complex, which recruits the 40S subunit to the 5' cap of mRNA. The two-domain RNA helicase eIF4A is a very weak helicase by itself, but the activity is enhanced upon interaction with the scaffolding protein eIF4G. Here we show that, albeit both eIF4A domains play a role in binding the middle domain of eIF4G (eIF4G-m, amino acids 745-1003), the main interaction surface is located on the C-terminal domain. We use NMR spectroscopy to define the binding site and find that the contact surface is adjacent to the RNA-, ATP-, and eIF4A-NTD-interacting regions. Mutations of interface residues abrogated binding, confirmed the interface, and showed that the N-terminal end of eIF4G-m interacts with the C-terminal domain of eIF4A. The data suggest that eIF4G-m forms a soft clamp to stabilize the closed interdomain orientation of eIF4A. This model can explain the cooperativity between all binding partners of eIF4A (eIF4G, RNA, ATP) and stimulation of eIF4A activity in the eIF4F complex.
Figures
Figure 1.
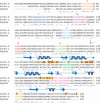
Binding of eIF4G-m to eIF4A detected by pull-down assays and NMR spectroscopy. (A) Lanes _1_-6 depict the His-tag pull-down experiments when immobilized His8-eIF4A-CTD (lanes _1_-3) and His6-eIF4A (lanes _4_-6) were incubated with eIF4G-m (lanes 1,4). (Lanes 2,5) Unbound eIF4G-m was eluted completely by extensive washing steps. Bound eIF4G-m was eluted as a complex with eIF4A-CTD (lane 3) and eIF4A (lane 6). Lanes _7_-12 show the reverse pull-down when untagged eIF4A-NTD was incubated on a control resin (lanes _7_-9) and a resin with immobilized His6-eIF4G-m (lanes _10_-12). After washing (lanes 8,11), bound proteins were eluted (lanes 9,12). (B) A characteristic overlay of 1H-15N-HSQC spectra of 15N-labeled eIF4A-CTD (500 μM) in free form (black) and complexed with the middle domain eIF4G-m (600 μM, red). (C) Overlays of 1H-15N-HSQC spectra of free 15N-labeled eIF4G-m (330 μM, blue) and 1:1 complexes of 15N-labeled eIF4G-m (130 μM, magenta) with equimolar amounts of full-length eIF4A (left), eIF4A-NTD (middle), and eIF4A-CTD (right) in buffer containing 300 mM NaCl. All spectra were acquired with the same number of scans. The intensities in the spectra of free 15N-labeled eIF4G-m were scaled to account for the difference in concentration. (D) 1D cross-sections of two-dimensional (2D) 1H-15N-HSQC spectra of free and complexed eIF4G-m taken at 119 ppm. The top and bottom panels show 1D slices as observed during titrations with buffer containing 300 and 100 mM NaCl, respectively. The different colors correspond to free eIF4G-m (black) and to eIF4G-m in complex with eIF4A-NTD (magenta), with eIF4A-CTD (cyan), and with eIF4A (navy).
Figure 2.
Sequence alignments of human eIF4A-1 (accession no. P60842), yeast eIF4A (accession no. P10081), and MjDEAD (PDB entry 1HV8), the homologous protein from the hyperthermophile Methanococcus jannaschii. The conserved motifs of the DEAD-box helicase are labeled and depicted in different colors (Q, orange; I, navy; Ia, purple; Ib, light blue; II, red; III, pale green; IV, dark green; V, magenta; VI, yellow) and labeled with roman numerals. Identical amino acids are indicated with (★); residues with conserved and semi-conserved substitutions are indicated with (:) and (.), respectively. The start site of the C-terminal construct of eIF4A-CTD is indicated with a red arrow. Secondary structure elements of yeast eIF4A are blue (arrow, β-strand; helix, α-helix). The nomenclature for the secondary structure elements (E1-E7) as used throughout the text is printed in blue at the beginning of the respective sheets and helices. Residues for which a decrease in the signal is observed after addition of eIF4G-m at eIF4A-CTD/eIF4G-m ratio of 3:1 are highlighted with orange boxes. Yellow boxes indicate additionally affected residues if the entire titration range (up to eIF4A-CTD/eIF4G-m ratio of 1:1.5) is taken into account. The sites of the mutations D265R/E268K and D296A/T298K are highlighted with (Δ). Residues with relaxation contribution resulting from conformational exchange are depicted with (∼).
Figure 3.
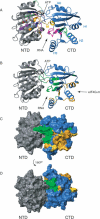
Structural model of full-length eIF4A and the eIF4G-m-binding regions on eIF4A-CTD. (A) Ribbon diagram of the homology model of full-length eIF4A. The N-terminal domain is colored gray; the C-terminal domain is colored blue. The conserved motifs are colored corresponding with Figure 2 (Q, orange; I, navy; Ia, purple; Ib, light blue; II, red; III, pale green; IV, dark green; V, magenta; VI, yellow). The position of motifs IV, V, and VI in eIF4A-CTD are highlighted with roman numerals. The faces of the interdomain clefts for substrate (RNA) and ATP binding are indicated with arrows. (B) Structural model of full-length eIF4A in the same orientation as in A. Residues involved in the binding to eIF4G-m are highlighted orange and gold; dynamic residues corresponding to very weak peaks and residues with exchange broadening are green (with the exceptions of R319 and G361, which are also affected by eIF4G-m binding and thus are colored orange). Surface representations in the same orientation (C) and after a 180° rotation (D) with the same color coding clearly show the distinct area involved in eIF4G-m binding.
Figure 4.
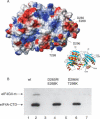
(A) Electrostatic surface of eIF4A with the locations of the four mutated residues in the double mutants D265R/E268K and D296A/T298K indicated by arrows. The orientation is shown in the insert on the right. (B) eIF4G-m binding to wild-type eIF4A-CTD and to the double mutants D265R/E268K and D296A/T298K detected by pull-down assays. Wild-type and mutant eIF4A-CTD protein was immobilized on a His-resin and incubated with eIF4G-m. (Lanes 1,3,5) Unbound eIF4G-m was eluted completely by extensive washing steps. Bound eIF4G-m was eluted as a complex with eIF4A-CTD wild type (wt) (lane 2), but no binding to eIF4G-m could be detected in the elution fractions of eIF4A-CTD D265R/E268K (lane 4) and eIF4A-CTD D296A/T298K (lane 6).
Figure 5.
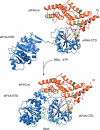
Model of the eIF4G-m as a soft clamp of eIF4A. We propose that eIF4G-m (orange) binds to the C-terminal domain of eIF4A (eIF4A-CTD, blue) and stabilizes the closed conformation of the helicase. Accessibility of the RNA- and ATP-binding surfaces of the helicase is retained. Residues contributing to the eIF4G-m-binding surface of eIF4A-CTD are colored gold. Surface mutations in eIF4G-m abolishing/drastically reducing binding of eIF4A and RNA are colored blue and green, respectively. Surface mutations that showed no effect are yellow. The open and closed conformations in our model correspond to the interdomain orientation as observed in the crystal structures of yeast eIF4A and MjDEAD (Caruthers et al. 2000; Story et al. 2001). To model the binding site of the oligonucleotide, human eIF4A-CTD from our homology model was superimposed to the structure of HCV NS3 in complex with bound oligonucleotide (Kim et al. 1998). For the sake of clarity, the structure of HCV NS3 is omitted and only the orientation of the oligonucleotide is shown. The SO4- ion found in the loop of the ATP-binding motif I is indicated by a filled circle.
Similar articles
- Crystal structure of the yeast eIF4A-eIF4G complex: an RNA-helicase controlled by protein-protein interactions.
Schütz P, Bumann M, Oberholzer AE, Bieniossek C, Trachsel H, Altmann M, Baumann U. Schütz P, et al. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9564-9. doi: 10.1073/pnas.0800418105. Epub 2008 Jul 7. Proc Natl Acad Sci U S A. 2008. PMID: 18606994 Free PMC article. - Two structurally atypical HEAT domains in the C-terminal portion of human eIF4G support binding to eIF4A and Mnk1.
Bellsolell L, Cho-Park PF, Poulin F, Sonenberg N, Burley SK. Bellsolell L, et al. Structure. 2006 May;14(5):913-23. doi: 10.1016/j.str.2006.03.012. Structure. 2006. PMID: 16698552 - Human translation initiation factor eIF4G1 possesses a low-affinity ATP binding site facing the ATP-binding cleft of eIF4A in the eIF4G/eIF4A complex.
Akabayov SR, Akabayov B, Wagner G. Akabayov SR, et al. Biochemistry. 2014 Oct 21;53(41):6422-5. doi: 10.1021/bi500600m. Epub 2014 Oct 8. Biochemistry. 2014. PMID: 25255371 Free PMC article. - Manipulation of the host translation initiation complex eIF4F by DNA viruses.
Walsh D. Walsh D. Biochem Soc Trans. 2010 Dec;38(6):1511-6. doi: 10.1042/BST0381511. Biochem Soc Trans. 2010. PMID: 21118117 Review. - Conducting the initiation of protein synthesis: the role of eIF4G.
Prévôt D, Darlix JL, Ohlmann T. Prévôt D, et al. Biol Cell. 2003 May-Jun;95(3-4):141-56. doi: 10.1016/s0248-4900(03)00031-5. Biol Cell. 2003. PMID: 12867079 Review.
Cited by
- Cofactor-dependent specificity of a DEAD-box protein.
Young CL, Khoshnevis S, Karbstein K. Young CL, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):E2668-76. doi: 10.1073/pnas.1302577110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630256 Free PMC article. - Molecular mechanism of scanning and start codon selection in eukaryotes.
Hinnebusch AG. Hinnebusch AG. Microbiol Mol Biol Rev. 2011 Sep;75(3):434-67, first page of table of contents. doi: 10.1128/MMBR.00008-11. Microbiol Mol Biol Rev. 2011. PMID: 21885680 Free PMC article. Review. - Interactions between eIF4AI and its accessory factors eIF4B and eIF4H.
Rozovsky N, Butterworth AC, Moore MJ. Rozovsky N, et al. RNA. 2008 Oct;14(10):2136-48. doi: 10.1261/rna.1049608. Epub 2008 Aug 21. RNA. 2008. PMID: 18719248 Free PMC article. - Getting the message in protein synthesis. Keystone Symposium on Translational Regulatory Mechanisms.
Costa-Mattioli M, Bidinosti M, Dever TE. Costa-Mattioli M, et al. EMBO Rep. 2008 Oct;9(10):954-9. doi: 10.1038/embor.2008.165. Epub 2008 Aug 29. EMBO Rep. 2008. PMID: 18758437 Free PMC article. Review. No abstract available. - Broad anti-pathogen potential of DEAD box RNA helicase eIF4A-targeting rocaglates.
Obermann W, Azri MFD, Konopka L, Schmidt N, Magari F, Sherman J, Silva LMR, Hermosilla C, Ludewig AH, Houhou H, Haeberlein S, Luo MY, Häcker I, Schetelig MF, Grevelding CG, Schroeder FC, Lau GSK, Taubert A, Rodriguez A, Heine A, Yeo TC, Grünweller A, Taroncher-Oldenburg G. Obermann W, et al. Sci Rep. 2023 Jun 8;13(1):9297. doi: 10.1038/s41598-023-35765-6. Sci Rep. 2023. PMID: 37291191 Free PMC article.
References
- Abramson R.D., Dever, T.E., and Merrick, W.C. 1988. Biochemical evidence supporting a mechanism for cap-independent and internal initiation of eukaryotic mRNA. J. Biol. Chem. 263: 6016-6019. - PubMed
- Ali I.K. and Jackson, R.J. 2001. The translation of capped mRNAs has an absolute requirement for the central domain of eIF4G but not for the cap-binding initiation factor eIF4E. Cold Spring Harb. Symp. Quant. Biol. 66: 377-387. - PubMed
- Bartels C., Xia, T., Billeter, M., Güntert, P., and Wüthrich, K. 1995. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 5: 1-10. - PubMed
- Benz J., Trachsel, H., and Baumann, U. 1999. Crystal structure of the ATPase domain of translation initiation factor 4A from Saccharomyces cerevisiae: The prototype of the DEAD box protein family. Structure Fold. Des. 7: 671-679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous