Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons - PubMed (original) (raw)
Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons
Hoon Ryu et al. Proc Natl Acad Sci U S A. 2005.
Abstract
The protein kinase A (PKA) and the cAMP response element (CRE) binding protein (CREB) signaling pathways mediate plasticity and prosurvival responses in neurons through their ability to regulate gene expression. The PKA-CREB signaling mechanism has been well characterized in terms of nuclear gene expression. We show that the PKA catalytic and regulatory subunits and CREB are localized to the mitochondrial matrix of neurons. Mitochondrial CRE sites were identified by using both serial analyses of chromatin occupancy and chromatin immunoprecipitation. Deferoxamine (DFO), an antioxidant and iron chelator known to inhibit oxidative stress-induced death, activated mitochondrial PKA and increased mitochondrial CREB phosphorylation (Ser-133). DFO increased CREB binding to CRE in the mitochondrial D-loop DNA and D-loop CRE-driven luciferase activity. In contrast, KT5720, a specific inhibitor of PKA, reduced DFO-mediated neuronal survival against oxidative stress induced by glutathione depletion. Neuronal survival by DFO may be, in part, mediated by the mitochondrial PKA-dependent pathway. These results suggest that the regulation of mitochondrial function via the mitochondrial PKA and CREB pathways may underlie some of the salutary effects of DFO in neurons.
Figures
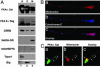
Fig. 1.
Mitochondrial localization of PKA-Cat and PKA-Reg subunits. (A) Ultrafractionation of cellular compartments confirmed that PKA-Cat and PKA-Reg subunits are present in pure mitochondrial fractions in mouse brain tissues. PKA-Cat and PKA-Reg subunits were detected at 36- and 43-kDa positions, respectively. The same blot was stripped and reblotted with anti-mitochondrial HSP70 and CREB antibody. N, nuclear fraction; C, cytoplasmic fraction; M, pure mitochondrial fraction. (B) Mitochondria are shown by colocalization of MitoTracker (a, red color) and cytochrome c (b, blue color) in a single neuron. (c) The image (pink color) is derived from superimposing the fluorescence of both a and b. (C) Punctate structures of PKA-Cat (a) are colocalized with MitoTracker (b). White arrows indicate colocalization of PKA and MitoTracker in the perinuclear region and dendritic segment in a single neuron. (c) The overlay image of a and b..
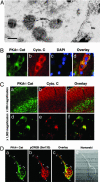
Fig. 2.
PKA-Cat is localized in the mitochondrial matrix in neurons. (A) Immunogold particles of PKA-Cat are present in the mitochondrial matrix in neurons. Gold particles are seen along portions of mitochondria (M). PKA-Cat visualization in embryonic day 17 rat cortical neurons by immunogold labeling was processed by using anti-PKA polyclonal antibody at 1:10 dilution. (Scale bar: 20 nm.) (B) PKA-Cat (a, green color) colocalizes with cytochrome c (b, red color) in human cerebral cortex neurons. (c) Nuclei are stained with DAPI. (d) The image is derived from superimposing the fluorescence images of a_–_c. (Scale bar: 10 μm.) (C) PKA-Cat (a and d, green) colocalizes with cytchrome c (b and e, red) in adult rat brain. c and f are overlay images. (D) PKA colocalizes with pCREB in cortical neurons. Cultures were double-stained for PKA (a) and pCREB (b). Arrows indicate punctate structures of colocalization of PKA and pCREB. (c) An overlay of a and b. (d) Nomarski microscopy of neuron observed in a_–_c.
Fig. 3.
DFO induces mitochondrial PKA activation and mitochondrial CREB phosphorylation. (A) DFO transiently increases the level of the phosphorylated Reg subunit II of PKA (PKA RII) in primary cortical neurons. NADH-oxidoreductase (NADH-OR) was detected as a control of protein level in the mitochondrial fraction. (B) DFO induces a transient phosphorylation of mitochondrial CREB. (C) Dose–response relationships for the phosphorylation of CREB by DFO in the mitochondrial fraction (n = 5). pCREB blot was stripped and reprobed with anti-CREB polyclonal (240) antibody. (D) The level of cytochrome c was not changed by DFO (n = 3). (E) In vitro mitochondrial pCREB staining was measured by flow cytometry and confirmed that mitochondrial pCREB was increased by DFO (100 μM). (F) Other iron chelators, mimosine (MIMO) and cobalt chloride (CoCl2), induced the phosphorylation of mitochondrial CREB. CONT, control. (G) Inhibition of PKA blocked the phosphorylation of mitochondrial CREB by DFO.
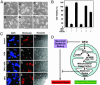
Fig. 4.
Confirmation of CREB binding sites in mitochondrial genome of PC12 cells by SACO and ChIP. (A) A diagram depicting the clustering of CREB SACO tags in the rat mitochondrial D-loop region and the adjacent cytochrome B gene. The positions of tags and genes in the mitochondrial genome is based on National Center for Biotechnology Information accession no. NC001665. (B) A summary of rat mitochondrial CREB loci based on their position, sequence, and number of tags identified. The labels on the left indicate the mitochondrial genes in which the tags cluster. Sequences represent L-strand of rat mtDNA. Cyt B, cytochrome B; ND 4 and 5, NADH dehydrogenase 4 and 5, ATP6, ATPase 6; COX1, complex 1. (C) PC12 cells were subjected to a ChIP assay using an anti-CREB antibody or nonspecific IgG as described in Materials and Methods. Levels of immunoprecipitated DNA encompassing the mitochondrial D-loop or the ND2 gene were assessed by real-time quantitative PCR. Data from triplicate determinations were normalized for amplicon size. Error bars denote SEM.
Fig. 5.
DFO modulates CREB binding to D-loop CRE sequence, D-loop CRE-reporter activity, and mitochondrial function. (A) Mouse and rat mtDNA contain CRE-like site. (B) Purified CREB binds to a mitochondrial CRE in the D-loop region of mitochondrial genome. I; mouse mitochondrial CRE, II; rat mitochondrial CRE; NC, negative control probe (L-strand, 5′-TGGCCACAGCACTTAAAG-3′). (C) Mitochondrial fractions from rat brain show mitochondrial CRE (rat) binding activity. Supershift (SS) analysis using a pCREB-specific antibody confirmed the specificity of CREB binding. NS, nonspecific bindings. (D) DFO increases CREB DNA binding activity to rat mitochondrial CRE. The mitochondrial fraction of rat primary cortical neurons was used for EMSA in vitro. (E) DFO modulates in vivo CREB binding to the mitochondrial D-loop DNA in mouse primary cultures. A modified ChIP method was used for detection of in vivo binding of CREB to mtDNA as described in Materials and Methods. (F) Construction of mitochondrial CRE-driven reporter vectors. (G) PKA inhibition abrogates DFO-induced mitochondrial CRE reporter activity. (H) DFO regulates CREB phosphorylation-dependent mitochondrial CRE-reporter activities. HT-22 cells were transiently transfected with pGL3E and pGL3E–D-loop CRE with WT or mutant (mt) CREB. mt1, Ser-133A CREB; mt2, Ser-142/144A CREB. Luciferase activity was normalized to the protein concentration of each sample. Data are the mean ± SE of three separate experiments.
Fig. 6.
PKA pathway is involved in neuronal protection by DFO. (A) Cortical neurons treated with HCA, DFO, and KT5720. Cells were pretreated with KT5720 (10 μM) 30 min before DFO (100 μM) treatment. (a) Control. (b) HCA. (c) DFO plus HCA. (d) KT5720 plus DFO. (e) KT5720 plus DFO plus HCA. (f) KT5720. (B) The PKA inhibitor KT5720 partially abrogates the protective function of DFO in HCA-treated cortical neurons. (C) DFO prevents loss of mitochondrial membrane potential induced by oxidative stress in cortical neurons. Cortical neuron cultures were exposed to 1 mM of HCA for 18 h with or without DFO (100 μM). Cultures were stained for nucleus with DAPI (a, d, g, and j) and for mitochondria with MitoTracker (b, e, h, and k). (a_–_c) Control. (d_–_f) HCA treatment. (g_–_i) DFO plus HCA treatment. (j_–_l) DFO treatment. White arrows in d, e, and f indicate the apoptotic cells induced by HCA. (c, f, i, and l) The Nomarski view of cortical neurons. (D) A proposed scheme of DFO-mediated neuronal survival through the mitochondrial PKA and CREB-dependent pathway.
Similar articles
- Cyclophilin D deficiency rescues Aβ-impaired PKA/CREB signaling and alleviates synaptic degeneration.
Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, Yan SS. Du H, et al. Biochim Biophys Acta. 2014 Dec;1842(12 Pt A):2517-27. doi: 10.1016/j.bbadis.2013.03.004. Epub 2013 Mar 16. Biochim Biophys Acta. 2014. PMID: 23507145 Free PMC article. - Astragaloside IV Protects Primary Cerebral Cortical Neurons from Oxygen and Glucose Deprivation/Reoxygenation by Activating the PKA/CREB Pathway.
Xue B, Huang J, Ma B, Yang B, Chang D, Liu J. Xue B, et al. Neuroscience. 2019 Apr 15;404:326-337. doi: 10.1016/j.neuroscience.2019.01.040. Epub 2019 Jan 30. Neuroscience. 2019. PMID: 30708047 - Prostaglandin E2 activates cAMP response element-binding protein in glioma cells via a signaling pathway involving PKA-dependent inhibition of ERK.
Bidwell P, Joh K, Leaver HA, Rizzo MT. Bidwell P, et al. Prostaglandins Other Lipid Mediat. 2010 Feb;91(1-2):18-29. doi: 10.1016/j.prostaglandins.2009.12.002. Epub 2009 Dec 14. Prostaglandins Other Lipid Mediat. 2010. PMID: 20015475
Cited by
- Cryptococcal Hsf3 controls intramitochondrial ROS homeostasis by regulating the respiratory process.
Gao X, Fu Y, Sun S, Gu T, Li Y, Sun T, Li H, Du W, Suo C, Li C, Gao Y, Meng Y, Ni Y, Yang S, Lan T, Sai S, Li J, Yu K, Wang P, Ding C. Gao X, et al. Nat Commun. 2022 Sep 15;13(1):5407. doi: 10.1038/s41467-022-33168-1. Nat Commun. 2022. PMID: 36109512 Free PMC article. - Phosphodiesterase Inhibitors for Alzheimer's Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale.
Sanders O, Rajagopal L. Sanders O, et al. J Alzheimers Dis Rep. 2020 Jun 16;4(1):185-215. doi: 10.3233/ADR-200191. J Alzheimers Dis Rep. 2020. PMID: 32715279 Free PMC article. Review. - Periodic 17β-estradiol pretreatment protects rat brain from cerebral ischemic damage via estrogen receptor-β.
Raval AP, Borges-Garcia R, Javier Moreno W, Perez-Pinzon MA, Bramlett H. Raval AP, et al. PLoS One. 2013 Apr 12;8(4):e60716. doi: 10.1371/journal.pone.0060716. Print 2013. PLoS One. 2013. PMID: 23593292 Free PMC article. - Modulation of SETDB1 activity by APQ ameliorates heterochromatin condensation, motor function, and neuropathology in a Huntington's disease mouse model.
Hwang YJ, Hyeon SJ, Kim Y, Lim S, Lee MY, Kim J, Londhe AM, Gotina L, Kim Y, Pae AN, Cho YS, Seong J, Seo H, Kim YK, Choo H, Ryu H, Min SJ. Hwang YJ, et al. J Enzyme Inhib Med Chem. 2021 Dec;36(1):856-868. doi: 10.1080/14756366.2021.1900160. J Enzyme Inhib Med Chem. 2021. PMID: 33771089 Free PMC article. - Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway.
Kim J, Kim SR, Choi YH, Shin JY, Kim CD, Kang NG, Park BC, Lee S. Kim J, et al. Molecules. 2020 Sep 2;25(17):4004. doi: 10.3390/molecules25174004. Molecules. 2020. PMID: 32887384 Free PMC article.
References
- Shaywitz, A. J. & Greenberg, M. E. (1999) Annu. Rev. Biochem. 68, 821–861. - PubMed
- Mayr, B. & Montminy, M. (2001) Nat. Rev. Mol. Cell. Biol. 8, 599–609. - PubMed
- Lonze, B. E. & Ginty, D. D. (2002) Neuron 35, 605–623. - PubMed
- Paolillo, M., Feliciello, A., Porcellini, A., Garbi, C., Bifulco, M., Schinelli, S., Ventra, C., Stabile, E., Ricciardelli, G., Schettini, G. & Avvedimento, E. V. (1999) J. Biol. Chem. 274, 6546–6552. - PubMed
- Riccio, A., Ahn, S., Davenport, C. M., Blendy, J. A. & Ginty, D. D. (1999) Science 286, 2358–2361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG013846/AG/NIA NIH HHS/United States
- NS40591/NS/NINDS NIH HHS/United States
- R01 NS052724/NS/NINDS NIH HHS/United States
- NS045806/NS/NINDS NIH HHS/United States
- NS52724-01/NS/NINDS NIH HHS/United States
- R01 NS040591/NS/NINDS NIH HHS/United States
- P30 AG13846/AG/NIA NIH HHS/United States
- U01 NS045806/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases