The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts - PubMed (original) (raw)
Comparative Study
The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts
Nathan T Ihle et al. Mol Cancer Ther. 2005 Sep.
Abstract
Epidermal growth factor receptor (EGFR) inhibitors such as gefitinib show antitumor activity in a subset of non-small cell lung cancer (NSCLC) patients having mutated EGFR. Recent work shows that phosphatidylinositol-3-kinase (PI3-K) is coupled to the EGFR only in NSCLC cell lines expressing ErbB-3 and that EGFR inhibitors do not inhibit PI3-K signaling in these cells. The central role PI3-K plays in cell survival suggests that a PI3-K inhibitor offers a strategy to increase the antitumor activity of EGFR inhibitors in resistant NSCL tumors that do not express ErbB-3. We show that PX-866, a PI3-K inhibitor with selectivity for p110alpha, potentiates the antitumor activity of gefitinib against even large A-549 NSCL xenografts giving complete tumor growth control in the early stages of treatment. A-549 xenograft phospho-Akt was inhibited by PX-866 but not by gefitinib. A major toxicity of PX-866 administration was hyperglycemia with decreased glucose tolerance, which was reversed upon cessation of treatment. The decreased glucose tolerance caused by PX-866 was insensitive to the AMP-activated protein kinase inhibitor metformin but reversed by insulin and by the peroxisome proliferator-activated receptor-gamma activator pioglitazone. Prolonged PX-866 administration also caused increased neutrophil counts. Thus, PX-866, by inhibiting PI3-K signaling, may have clinical use in increasing the response to EGFR inhibitors such as gefitinib in patients with NSCLC and possibly in other cancers who do not respond to EGFR inhibition.
Figures
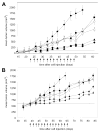
Figure 1. Potentiation of the antitumor activity of gefitnib by PX-866
Female scid mice were implanted subcutaneously with 107 A-549 human non small cell lung cancer cells. A, Tumors were 100 mm3 on day 22 (shown by dashed line) when dosing was begun every other day for 15 doses shown by the arrows with () vehicle alone; (_) gefitinib 75 mg/kg po; (Δ) PX-866 9 mg/kg iv; (_) PX-866 2.5 mg/kg po; (_) PX-866 9 mg/kg iv 4 hr before gefitinib 75 mg/kg po; (_) PX-866 2.5 mg/kg po 4 hr before gefitinib 75 mg/kg po. Values are the mean of 8 mice per group and bars are SE. B. Tumors were 600 mm3 on day 39 (shown by dashed line) when dosing was begun every other day for 14 doses shown by the arrows with (_ ) vehicle alone; (_) gefitinib 75 mg/kg po; (Δ) PX-866 12 mg/kg iv; (_) PX-866 4 mg/kg po; (_) PX-866 12 mg/kg iv 4 hr before gefitinib 75 mg/kg po; (_) PX-866 4 mg/kg po 4 hr before gefitinib 75 mg/kg po. Values are the mean of 8 mice per group and bars are SE.
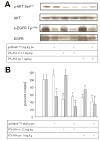
Figure 2
Inhibition of EGFR and phospho-Akt in A-549 non small cell lung cancer xenografts by gefitinib and PX-866.. Female scid mice with 300 mm3 A-549 non small cell lung cancer xenografts were administered gefitinib 75 mg/kg po, PX-866 12 mg/kg iv or PX-866 3 mg/kg po, alone or with the PX-866 4 hr before the gefitinib, daily every other day for 5 days. 24 hr after the last dose tumors were removed for measurement of phospho-Akt/total Akt shown by the open bars or phospho-EGFR shown by the shaded bars. Values are the mean of 3 mice and bars are SE. * p < 0.05 compared to non treated control.
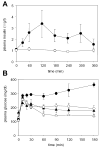
Figure 3. Effect of PX-866 on plasma insulin and glucose tolerance
A, Plasma insulin in female C57Bl/6 mice fasted for 16 hr. (○) vehicle control and (•) mice administered PX-866 10 mg/kg po. Values are the mean of 3 mice and bars are SE. B, Plasma glucose in female C57Bl/6 mice fasted for 16 hr and administered a dose of glucose of 1 g/kg po. (○) vehicle control; (•) administered PX-866 10 mg/kg 4 hr previously; (_) treated daily for 7 days with pioglitazone 10 mg/kg ip; and (Δ) treated daily for 7 days with pioglitazone 10 mg/kg ip and administered PX-866 10 mg/kg 4 hr previously. Values are the mean of 4 mice and bars are SE.
Similar articles
- Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy.
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY, Chen JJ, Chen HW, Yang PC. Lee JY, et al. PLoS One. 2011;6(8):e23756. doi: 10.1371/journal.pone.0023756. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858220 Free PMC article. - Peroxisome proliferator-activated receptor gamma agonist pioglitazone prevents the hyperglycemia caused by phosphatidylinositol 3-kinase pathway inhibition by PX-866 without affecting antitumor activity.
Ihle NT, Lemos R, Schwartz D, Oh J, Halter RJ, Wipf P, Kirkpatrick L, Powis G. Ihle NT, et al. Mol Cancer Ther. 2009 Jan;8(1):94-100. doi: 10.1158/1535-7163.MCT-08-0714. Mol Cancer Ther. 2009. PMID: 19139117 Free PMC article. - A novel STAT3 inhibitor W2014-S regresses human non-small cell lung cancer xenografts and sensitizes EGFR-TKI acquired resistance.
Zheng Q, Dong H, Mo J, Zhang Y, Huang J, Ouyang S, Shi S, Zhu K, Qu X, Hu W, Liu P, Wang Y, Zhang X. Zheng Q, et al. Theranostics. 2021 Jan 1;11(2):824-840. doi: 10.7150/thno.49600. eCollection 2021. Theranostics. 2021. PMID: 33391507 Free PMC article. - Inhibition of the epidermal growth factor receptor in combined modality treatment for locally advanced non-small cell lung cancer.
Ready N. Ready N. Semin Oncol. 2005 Apr;32(2 Suppl 3):S35-41. doi: 10.1053/j.seminoncol.2005.03.008. Semin Oncol. 2005. PMID: 16015534 Review. - Gefitinib in lung cancer therapy: clinical results, predictive markers of response and future perspectives.
Adamo V, Franchina T, Adamo B, Denaro N, Gambadauro P, Chiofalo G, Scimone A, Caristi N, Russo A, Giordano A. Adamo V, et al. Cancer Biol Ther. 2009 Feb;8(3):206-12. doi: 10.4161/cbt.8.3.7465. Epub 2009 Feb 20. Cancer Biol Ther. 2009. PMID: 19182534 Review.
Cited by
- The Biological Role of PI3K Pathway in Lung Cancer.
Sarris EG, Saif MW, Syrigos KN. Sarris EG, et al. Pharmaceuticals (Basel). 2012 Nov 20;5(11):1236-64. doi: 10.3390/ph5111236. Pharmaceuticals (Basel). 2012. PMID: 24281308 Free PMC article. - Targeting PI3K family with small-molecule inhibitors in cancer therapy: current clinical status and future directions.
Li H, Wen X, Ren Y, Fan Z, Zhang J, He G, Fu L. Li H, et al. Mol Cancer. 2024 Aug 10;23(1):164. doi: 10.1186/s12943-024-02072-1. Mol Cancer. 2024. PMID: 39127670 Free PMC article. Review. - Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations.
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. LoPiccolo J, et al. Drug Resist Updat. 2008 Feb-Apr;11(1-2):32-50. doi: 10.1016/j.drup.2007.11.003. Epub 2007 Dec 31. Drug Resist Updat. 2008. PMID: 18166498 Free PMC article. Review. - Selective Raf inhibition in cancer therapy.
Khazak V, Astsaturov I, Serebriiskii IG, Golemis EA. Khazak V, et al. Expert Opin Ther Targets. 2007 Dec;11(12):1587-609. doi: 10.1517/14728222.11.12.1587. Expert Opin Ther Targets. 2007. PMID: 18020980 Free PMC article. Review. - The PI3K pathway as drug target in human cancer.
Courtney KD, Corcoran RB, Engelman JA. Courtney KD, et al. J Clin Oncol. 2010 Feb 20;28(6):1075-83. doi: 10.1200/JCO.2009.25.3641. Epub 2010 Jan 19. J Clin Oncol. 2010. PMID: 20085938 Free PMC article. Review.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2000;296:1655–1657. - PubMed
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
- Hill MM, Feng J, Hemmings BA. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12:1251–1255. - PubMed
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signaling. 2002;14:381–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA052995/CA/NCI NIH HHS/United States
- U19 CA052995/CA/NCI NIH HHS/United States
- U54 CA090821/CA/NCI NIH HHS/United States
- CA90821/CA/NCI NIH HHS/United States
- CA52995/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous