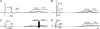Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion - PubMed (original) (raw)
Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion
Fletcher A White et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Neuronal hyperexcitability in both injured and adjacent uninjured neurons is associated with states of chronic injury and pain and is likely subject to neuroinflammatory processes. Chronic inflammatory responses are largely orchestrated by chemokines. One chemokine, monocyte chemoattractant protein-1 (MCP-1), in the presence of its cognate receptor, the beta chemokine receptor 2 (CCR2), produces neural activity in dissociated neuronal cultures of neonatal dorsal root ganglion (DRG) neurons. Using a neuropathic pain model, chronic compression of the DRG (CCD), we compared anatomically separate populations of noncompressed lumbar DRG (L3/L6) with compressed lumbar DRG (L4/L5) for changes in the gene expression of CCR2. In situ hybridization revealed that CCR2 mRNA was up-regulated in neurons and nonneuronal cells present in both compressed L4/L5 and ipsilateral noncompressed L3/L6 DRGs at postoperative day 5 (POD5). The total percentages of compressed and noncompressed neurons exhibiting CCR2 mRNA transcripts in L3, L5, and L6 DRG were 33 +/- 3.5%, 49 +/- 6.2%, and 41 +/- 5.6%, respectively, and included cell bodies of small, medium, and large size. In addition, the preferred CCR2 ligand, MCP-1, was up-regulated by POD5 in both compressed L4/L5 and noncompressed L3/L6 DRG neurons. Application of MCP-1 to the cell bodies of the intact formerly compressed DRG in vitro produced potent excitatory effects not observed in control ganglia. MCP-1/CCR2 signaling is directly involved with a chronic compression injury and may contribute to associated neuronal hyperexcitability and neuropathic pain.
Figures
Fig. 1.
CCR2 mRNA expression in compressed and adjacent noncompressed DRG at POD1, POD3, and POD5. (A) Sense riboprobe hybridization signal in representative compressed L5 DRG at POD5. (B and C) Compressed L5 DRG (B) and adjacent noncompressed L6 DRG (C) do not exhibit CCR2 mRNA expression at POD1. (D) Sham-treated L5 DRG expression levels of CCR2 mRNA at POD5. (E and F) Compressed L5 DRG (E) and adjacent noncompressed L6 (F) exhibit only neuronal CCR2 mRNA expression (white arrows) at POD3. (G–I) High levels of CCR2 mRNA are present in predominantly nonneuronal cells and some neurons (white arrows) of adjacent noncompressed L3 (G) and L6 (I) DRG at POD5. Compressed L5 DRG (H) at POD5 also exhibit high levels of CCR2 mRNA expression in predominantly nonneuronal cells, but many more neurons are positive for CCR2 mRNA transcripts (white arrows). Black asterisks indicate nonlabeled neurons (G–I). (Bar, 50 μm.)
Fig. 2.
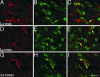
MCP-1 immunoreactivity and isolectin B4 binding in compressed lumbar DRG 5 (L5) and adjacent noncompressed L6 DRG at POD5 and in compressed L5 ganglia at POD24. (A, D, and G) MCP-1 immunoreactivity (red arrows) in (A) L5 and (D) L6 DRG at POD5, and (G) L5 DRG at POD24. (B, E, and H) Isolectin B4 binding (green arrows) in compressed L5 (B) and adjacent noncompressed L6 DRG (E) at POD5 and in compressed L5 DRG (H) at POD24. (C, F, and I) Merged images of MCP-1 (red arrows) and IB4 (green arrows) and colocalization (yellow arrows) at POD5 (C and F) and POD24 (I). (Bars, 100 μm.)
Fig. 3.
Responses of DRG neurons to MCP-1 applied to somata on the surface of the formerly compressed ganglion at POD5. The neurons were classified visually by somal size as small (A), medium (B and C), or large (D). Each neuron was further classified by axonal conduction velocity (action potential proceeded by artifact electrically evoked from dorsal root, on the left) and by the presence or absence of a “hump” on the falling phase of the action potential causing an extra deflection in the first derivative (arrows, A and B Left Inset). The hump is typical for nociceptive neurons
Similar articles
- Expression of monocyte chemoattractant protein-1 in rat dorsal root ganglia and spinal cord in experimental models of neuropathic pain.
Jeon SM, Lee KM, Cho HJ. Jeon SM, et al. Brain Res. 2009 Jan 28;1251:103-11. doi: 10.1016/j.brainres.2008.11.046. Epub 2008 Nov 27. Brain Res. 2009. PMID: 19059387 - Expression of monocyte chemoattractant protein-1 and its induction by tumor necrosis factor receptor 1 in sensory neurons in the ventral rhizotomy model of neuropathic pain.
Jeon SM, Sung JK, Cho HJ. Jeon SM, et al. Neuroscience. 2011 Sep 8;190:354-66. doi: 10.1016/j.neuroscience.2011.06.036. Epub 2011 Jun 25. Neuroscience. 2011. PMID: 21712071 - Contribution of degeneration of motor and sensory fibers to pain behavior and the changes in neurotrophic factors in rat dorsal root ganglion.
Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Obata K, et al. Exp Neurol. 2004 Jul;188(1):149-60. doi: 10.1016/j.expneurol.2004.03.012. Exp Neurol. 2004. PMID: 15191811 - Chemokine signaling and the management of neuropathic pain.
White FA, Feldman P, Miller RJ. White FA, et al. Mol Interv. 2009 Aug;9(4):188-95. doi: 10.1124/mi.9.4.7. Mol Interv. 2009. PMID: 19720751 Free PMC article. Review. - Chemokines and pain mechanisms.
Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Abbadie C, et al. Brain Res Rev. 2009 Apr;60(1):125-34. doi: 10.1016/j.brainresrev.2008.12.002. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19146875 Free PMC article. Review.
Cited by
- The Role of Cytokines and Chemokines in Shaping the Immune Microenvironment of Glioblastoma: Implications for Immunotherapy.
Yeo ECF, Brown MP, Gargett T, Ebert LM. Yeo ECF, et al. Cells. 2021 Mar 9;10(3):607. doi: 10.3390/cells10030607. Cells. 2021. PMID: 33803414 Free PMC article. Review. - The chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a Gβγ-dependent mechanism.
Belkouch M, Dansereau MA, Réaux-Le Goazigo A, Van Steenwinckel J, Beaudet N, Chraibi A, Melik-Parsadaniantz S, Sarret P. Belkouch M, et al. J Neurosci. 2011 Dec 14;31(50):18381-90. doi: 10.1523/JNEUROSCI.3386-11.2011. J Neurosci. 2011. PMID: 22171040 Free PMC article. - Differential transcriptional profiling of damaged and intact adjacent dorsal root ganglia neurons in neuropathic pain.
Reinhold AK, Batti L, Bilbao D, Buness A, Rittner HL, Heppenstall PA. Reinhold AK, et al. PLoS One. 2015 Apr 16;10(4):e0123342. doi: 10.1371/journal.pone.0123342. eCollection 2015. PLoS One. 2015. PMID: 25880204 Free PMC article. - Neuronal chemokines: versatile messengers in central nervous system cell interaction.
de Haas AH, van Weering HR, de Jong EK, Boddeke HW, Biber KP. de Haas AH, et al. Mol Neurobiol. 2007 Oct;36(2):137-51. doi: 10.1007/s12035-007-0036-8. Epub 2007 Jul 10. Mol Neurobiol. 2007. PMID: 17952658 Free PMC article. Review. - Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia.
Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Xie WR, et al. Neuroscience. 2006 Oct 27;142(3):809-22. doi: 10.1016/j.neuroscience.2006.06.045. Epub 2006 Aug 2. Neuroscience. 2006. PMID: 16887276 Free PMC article.
References
- Ma, C., Shu, Y., Zheng, Z., Chen, Y., Yao, H., Greenquist, K. W., White, F. A. & LaMotte, R. H. (2003) J. Neurophysiol. 89, 1588-1602. - PubMed
- Waters, S. M., Steflik, J., Cortright, D. N., Ma, C., LaMotte, R. H. & White, F. A. (2004) Soc. Neurosci. Abstr. 31, 64.4.
Publication types
MeSH terms
Substances
Grants and funding
- MH040165/MH/NIMH NIH HHS/United States
- NS014624/NS/NINDS NIH HHS/United States
- R01 NS049136/NS/NINDS NIH HHS/United States
- R01 DA013141/DA/NIDA NIH HHS/United States
- NS038317/NS/NINDS NIH HHS/United States
- DA01341/DA/NIDA NIH HHS/United States
- R37 MH040165/MH/NIMH NIH HHS/United States
- R01 NS014624/NS/NINDS NIH HHS/United States
- R01 NS043095/NS/NINDS NIH HHS/United States
- NS043095/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous