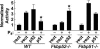Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation - PubMed (original) (raw)
Comparative Study
. 2005 Oct 4;102(40):14326-31.
doi: 10.1073/pnas.0505775102. Epub 2005 Sep 21.
Affiliations
- PMID: 16176985
- PMCID: PMC1242310
- DOI: 10.1073/pnas.0505775102
Comparative Study
Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation
Susanne Tranguch et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Embryo implantation in the uterus is a critical step in mammalian reproduction, requiring preparation of the uterus receptive to blastocyst implantation. Uterine receptivity, also known as the window of implantation, lasts for a limited period, and it is during this period blastocysts normally implant. Ovarian steroid hormones estrogen and progesterone (P(4)) are the primary regulators of this process. The immunophilin FKBP52 serves as a cochaperone for steroid hormone nuclear receptors to govern appropriate hormone action in target tissues. Here we show a critical role for FKBP52 in mouse implantation. This immunophilin has unique spatiotemporal expression in the uterus during implantation, and females missing the Fkbp52 gene have complete implantation failure due to lack of attainment of uterine receptivity. The overlapping uterine expression of FKBP52 with nuclear progesterone receptor (PR) in wild-type mice together with reduced P(4) binding to PR, attenuated PR transcriptional activity and down-regulation of several P(4)-regulated genes in uteri of Fkbp52(-/-) mice, establishes this cochaperone as a critical regulator of uterine P(4) function. Interestingly, ovulation, another P(4)-mediated event, remains normal. Collectively, the present investigation provides evidence for an in vivo role for this cochaperone in regulating tissue-specific hormone action and its critical role in uterine receptivity for implantation.
Figures
Fig. 1.
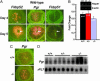
Female reproductive events in _Fkbp52_-/- mice. (A) Normal ovulation and fertilization were examined on day 2 of pregnancy. Superovulated eggs retrieved on day 1 were subjected to in vitro fertilization. Numbers within the bars indicate number of fertilized eggs/total number of ovulated eggs. Normal and superovulation rates are not significantly different between +/+ and -/- mice (unpaired t test); however, normal or in vitro fertilization rates are significantly different between +/+ and -/- mice (χ2 analysis; *, P = 0.01). (B) Fkbp52 and Pgr expression in preimplantation embryos as assessed by RT-PCR. (C) Representative photographs of wild-type uteri with implantation sites (IS) and _Fkbp52_-/- uteri without IS on day 5 of pregnancy. Arrowheads and arrows indicate sites of ovary and implantation, respectively. (D) Representative photomicrograph of blastocysts recovered from _Fkbp52_-/- mice on day 5 of pregnancy. (Bar, 50 μm.)
Fig. 2.
Uterine expression of Fkbp52, Pgr and Fkbp51. (A) In situ hybridization of Fkbp52, Pgr, and Fkbp51 in wild-type uteri on days 4 and 5 of pregnancy. (B) Comparative RT-PCR of uterine Fkbp51 in Fkbp52+/+ (+/+), Fkbp52+/- (+/-), and _Fkbp52_-/- (-/-) mice on day 4 of pregnancy. The data are presented as fold changes (mean ± SEM) of three to four independent RNA samples. Fold changes are not significantly different between +/+, +/-, or -/- mice (unpaired t test). (C) In situ hybridization of uterine Pgr in wild-type (+/+) and _Fkbp52_-/- (-/-) mice on day 4. (D) Northern blot analysis of uterine Pgr in +/+, +/-, and -/- mice on day 4. rPL7 is a housekeeping gene. Arrows in A indicate the location of blastocysts. le, luminal epithelium; ge, glandular epithelium; s, stroma. (Bar, 400 μm.)
Fig. 3.
Differential modulation of PR transactivation by FKBP52 and FKBP51. Fibroblast cell lines prepared from wild-type (WT), _Fkbp52_-/-, or _Fkbp51_-/- mouse embryos were cotransfected with four plasmids as described in Materials and Methods. Cells were treated with or without 0.5 nM P4 for 16 h. The bars indicate the activity (mean ± SD; n = 3) normalized to uninduced levels of luciferase activity in each cell background. In WT cells expressing endogenous FKBP52 and FKBP51, overexpression of FKBP52 show marginal increases in the reporter activity, whereas overexpression of FKBP51 decreases the reporter activity (*, P = 0.05). In _Fkbp52_-/- cells, P4-induced reporter activity is minimal but restored by exogenous FKBP52 (*, P = 0.05). In _Fkbp51_-/- cells retaining endogenous FKBP52, maximal reporter activity is induced; however, induced expression of FKBP51 decreases the activity. Statistical analysis was performed by using one-way ANOVA followed by paired t test.
Fig. 4.
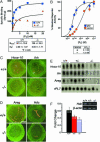
Uterine progesterone (P4) binding and PR activity. (A) P4 binding in uterine cytosol of wild-type (+/+) and Fkbp52-/- (-/-) mice. Both +/+ and -/- female mice were ovariectomized and treated with estrogen to increase uterine PR levels. Uterine cytosolic samples from +/+ and -/- mice were used for binding assays for comparison. The data shown are representative of four independent experiments. (B) FKBP52 effects on reporter gene activation in embryonic fibroblasts isolated from _Fkbp52_-/- mice cotransfected with plasmid expressing human PR-B, a luciferase reporter plasmid, and an empty vector or plasmid expressing FKBP52 (p52). Each value represents the mean ± SD for three replicate samples. (C and D) In situ hybridization of Hoxa-10 and Ihh (C) and Areg and Hdc (D) in +/+ and -/- mice on day 4 of pregnancy. (E) Northern blot analysis of uterine Hoxa-10, Ihh, and Areg in +/+, +/-, and -/- mice on day 4. rPL7 is a housekeeping gene. le, luminal epithelium; ge, glandular epithelium; s, stroma. (Bar, 400 μm.) (F) Comparative RT-PCR of uterine Hdc in +/+, +/-, and -/- mice on day 4. The data are presented as fold changes (mean ± SEM) of three to four independent samples. Fold changes in _Fkbp52_-/- mice are significantly different compared to combined fold changes in +/+ and +/- mice (P = 0.05, unpaired t test).
Fig. 5.
Differential expression of an estrogen-responsive gene lactoferrin (Ltf) and cell proliferation. (A) In situ hybridization of Ltf in uteri of +/+ and -/- mice on days 1 and 4 of pregnancy. (Bar, 400 μm.) (B) Histological examination of +/+ and -/- uteri on day 4 of pregnancy. (Bar, 200 μm.) (C) Immunostaining of Ki67, a marker of cell proliferation, in uteri of +/+ and -/- mice on day 4. Note stromal cell proliferation in wild-type uterus versus luminal epithelial proliferation in _Fkbp52_-/- mice. Arrow points to luminal epithelial layer. le, luminal epithelium; s, stroma; myo, myometrium. (Bar, 100 μm.)
Similar articles
- Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus.
Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Daikoku T, et al. Mol Endocrinol. 2005 Mar;19(3):683-97. doi: 10.1210/me.2004-0332. Epub 2004 Nov 4. Mol Endocrinol. 2005. PMID: 15528267 - FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform.
Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sánchez ER, Shou W. Yang Z, et al. Mol Endocrinol. 2006 Nov;20(11):2682-94. doi: 10.1210/me.2006-0024. Epub 2006 Jul 27. Mol Endocrinol. 2006. PMID: 16873445 Free PMC article. - Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress.
Hirota Y, Acar N, Tranguch S, Burnum KE, Xie H, Kodama A, Osuga Y, Ustunel I, Friedman DB, Caprioli RM, Daikoku T, Dey SK. Hirota Y, et al. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15577-82. doi: 10.1073/pnas.1009324107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713718 Free PMC article. - Progesterone receptor requires a co-chaperone for signalling in uterine biology and implantation.
Tranguch S, Smith DF, Dey SK. Tranguch S, et al. Reprod Biomed Online. 2006 Nov;13(5):651-60. doi: 10.1016/s1472-6483(10)60655-4. Reprod Biomed Online. 2006. PMID: 17169175 Review. - In vivo analysis of progesterone receptor action in the uterus during embryo implantation.
Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Franco HL, et al. Semin Cell Dev Biol. 2008 Apr;19(2):178-86. doi: 10.1016/j.semcdb.2007.12.001. Epub 2008 Jan 6. Semin Cell Dev Biol. 2008. PMID: 18280760 Review.
Cited by
- Epigenetic changes through DNA methylation contribute to uterine stromal cell decidualization.
Gao F, Ma X, Rusie A, Hemingway J, Ostmann AB, Chung D, Das SK. Gao F, et al. Endocrinology. 2012 Dec;153(12):6078-90. doi: 10.1210/en.2012-1457. Epub 2012 Oct 2. Endocrinology. 2012. PMID: 23033272 Free PMC article. - Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity.
Chen M, Zheng Z, Shi J, Shao J. Chen M, et al. Biomolecules. 2021 Dec 27;12(1):36. doi: 10.3390/biom12010036. Biomolecules. 2021. PMID: 35053184 Free PMC article. Review. - Glucocorticoids, their uses, sexual dimorphisms, and diseases: new concepts, mechanisms, and discoveries.
Martinez GJ, Appleton M, Kipp ZA, Loria AS, Min B, Hinds TD Jr. Martinez GJ, et al. Physiol Rev. 2024 Jan 1;104(1):473-532. doi: 10.1152/physrev.00021.2023. Epub 2023 Sep 21. Physiol Rev. 2024. PMID: 37732829 Free PMC article. Review. - The non-human primate model of endometriosis: research and implications for fecundity.
Braundmeier AG, Fazleabas AT. Braundmeier AG, et al. Mol Hum Reprod. 2009 Oct;15(10):577-86. doi: 10.1093/molehr/gap057. Epub 2009 Jul 24. Mol Hum Reprod. 2009. PMID: 19633013 Free PMC article. Review. - Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells.
De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, Chen Y, Ning YM, Riggs DL, Fletterick RJ, Guy RK, Trepel JB, Neckers LM, Cox MB. De Leon JT, et al. Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11878-83. doi: 10.1073/pnas.1105160108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730179 Free PMC article.
References
- Paria, B. C., Reese, J., Das, S. K. & Dey, S. K. (2002) Science 296, 2185-2188. - PubMed
- Dey, S. K., Lim, H., Das, S. K., Reese, J., Paria, B. C., Daikoku, T. & Wang, H. (2004) Endocr. Rev. 25, 341-373. - PubMed
- Mulac-Jericevic, B., Mullinax, R. A., DeMayo, F. J., Lydon, J. P. & Conneely, O. M. (2000) Science 289, 1751-1754. - PubMed
- Silverstein, A. M., Galigniana, M. D., Kanelakis, K. C., Radanyi, C., Renoir, J. M. & Pratt, W. B. (1999) J. Biol. Chem. 274, 36980-36986. - PubMed
- Barent, R. L., Nair, S. C., Carr, D. C., Ruan, Y., Rimerman, R. A., Fulton, J., Zhang, Y. & Smith, D. F. (1998) Mol. Endocrinol. 12, 342-354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous