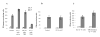Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells - PubMed (original) (raw)
Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells
Sergei Kusmartsev et al. J Immunol. 2005.
Abstract
T cell tolerance is a critical element of tumor escape. However, the mechanism of tumor-associated T cell tolerance remains unresolved. Using an experimental system utilizing the adoptive transfer of transgenic T cells into naive recipients, we found that the population of Gr-1+ immature myeloid cells (ImC) from tumor-bearing mice was able to induce CD8+ T cell tolerance. These ImC accumulate in large numbers in spleens, lymph nodes, and tumor tissues of tumor-bearing mice and are comprised of precursors of myeloid cells. Neither ImC from control mice nor progeny of tumor-derived ImC, including tumor-derived CD11c+ dendritic cells, were able to render T cells nonresponsive. ImC are able to take up soluble protein in vivo, process it, and present antigenic epitopes on their surface and induce Ag-specific T cell anergy. Thus, this is a first demonstration that in tumor-bearing mice CD8+ T cell tolerance is induced primarily by ImC that may have direct implications for cancer immunotherapy.
Figures
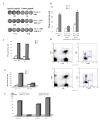
Figure 1. Induction of CD8 T cell non-responsiveness by adoptive transfer of Gr-1+ ImC derived from tumor-bearing host.
A. Adoptive transfer model. B. LN cells isolated from mice after adoptive transfer of OT-1 T cells were incubated in triplicates in the presence of specific (SIINFEKL) (S.P.) or control (RAHYNIVTF) (C.P.) peptides for 24 h. Number of IFN-γ producing cells was scored in ELISPOT assay. Results presented as Mean±SD. Six experiments with the same results were performed. C. Antigen specific IL-2 production. LN cells isolated from mice as described above were cultured in the presence of specific or control peptide for 24 hr. Cells were then labeled with anti-CD8-APC and anti-Valpha2- PE antibodies, and then, fixed, permeabilized and stained with anti-IL-2-FITC antibody. The percentage of IL-2 positive cells amongst the CD8+Vα2+ population is shown at the upper corner. Two experiments with the same results were performed. D. Antigen-specific proliferation. LN cells isolated from mice as described above were cultured for 4 days in triplicates in U-bottom 96-well plates (105 cells per well) in the presence of 10 μg/ml of specific or control peptide. Eighteen hours prior harvesting 1 μCi 3H-thymidine was added in each well and radioactivity was measured on liquid scintillation counter. Results presented as Mean±SD. E. LN cells isolated from mice after adoptive transfer of 2C T cells were incubated in triplicates in the presence of specific or control peptides for 24 h. Number of IFN-γ producing cells was evaluated in ELISPOT assay. Results presented as Mean±SD. Four experiments with the same results were performed. F. Cytotoxic activity in vivo. Splenocytes from syngeneic C57BL/6 mice loaded with fluorescent dye CFSE were used as targets. One group of splenocytes was pulsed for 2 hr with 10 μg/ml of the specific peptide (SIINFEKL), washed and loaded with 10 μM CFSE. The other group was pulsed with the control peptide (RAHYNIVTF) and loaded with 1 μM CFSE. Cells were then mixed together at 3:1 ratio and injected i.v. into mice 10 days after immunization and ImC administration as described in Fig. 1A. Thirty-six hours later spleens were collected and CFSE positive cells were evaluated. Left peak on histogram reflects the population of splenocytes pulsed with the control peptide, right peak – cells pulsed with the specific peptide. Two experiments with the same results were performed.
Figure 2. Antigen-specific nature of ImC mediated CD8+T-cell tolerance
A. Naïve mice recipients of OT-1 T cells were immunized with SIINFEKL. Gr-1+ cells were isolated from spleens of EL-4 tumor-bearing mice, pulsed with specific or control peptides and injected i.v. (3x106 cells) 8 days after immunization. LNs were collected 24 hr later. Cells were re-stimulated with control or specific peptides and IFN-γ production was evaluated in ELISPOT assay. Actual results of one experiment are shown. Numbers represent average of three wells. Three experiments with the same results were performed. B. OT-1 transgenic T cells (H2b) were transferred to F1 hybrid (C57BL/6(H2b) x BALB/c (H2d)) mice. Three days later these mice have received Gr-1+ cells from either EL-4 tumor-bearing C57BL/6 mice (H2b) or from MethA sarcoma-bearing BALB/c mice (H2d) and at the same time were immunized with SIINFEKL peptide. After 10 days LN cells from these mice were tested for production of IFN-γ in response to specific or control peptide using ELISPOT assay. Two independent experiments with the same results were performed. C. OT-1 T cells (3x106) were adoptively transferred into naïve recipient mice. Two days later these mice were injected with Gr-1+ cells (3x106) from EL-4 tumor-bearing mice and immunized s.c. with SIINFEKL peptide. Three days later LN cells were collected and stimulated with control or specific peptide as described in Fig. 1B. The number of IFN-γ producing cells was evaluated in ELISPOT assay. D. OT-1 T cells (3x106) were adoptively transferred into naïve recipient mice. Two days later these mice were injected with Gr-1+ cells (3x106) from EL-4 tumor-bearing mice and immunized s.c. with SIINFEKL peptide. Three days after immunization mice were injected i.p. with 1mg of BrdU. The next day regional LNs of immunized mice were isolated and cells were stained with anti-CD8-APC antibody, anti-Valpha2-PE antibody, anti-BrDU-FITC antibody and 7 AAD. CD8+Valpha+ cells were gated (left two panels) and BrDU positive proliferating cells were evaluated (gate # 1 in two right panels). E. OT-1 T cells (3x106) were adoptively transferred into naïve recipient mice. Two days later these mice were immunized s.c. with SIINFEKL peptide. Gr-1+ ImC isolated from EL-4 tumor-bearing mice were loaded (2 hr incubation at 37°C) with control (C.P.) or specific (S.P.) peptides, washed and then injected i.v. (3x106 cells). Three days later LN cells were collected and the number of IFN-γ producing cells was evaluated in ELISPOT assay. F. After adoptive transfer of OT-1 cells mice were immunized with either s.c. injection of specific peptide (SIINFEKL) with IFA or with 2x105 DCs pulsed with specific peptide. ImC (3x106 cells) were injected i.v. at the time of immunization. LN were collected 10 days after immunization and cells were stimulated with control or specific peptides as described above. The number of IFN-γ producing cells was measured in ELISPOT assay. To simplify the presentation of the data the values of IFN-γ producing cells after stimulation with control peptide were subtracted from the values of IFN-γ producing cells after stimulation with specific peptide.
Figure 3. The nature of cells responsible for ImC mediated CD8+ T cell tolerance
A. Experiments were performed as described in Fig. 1A. Gr-1+ cells were isolated from spleens of naïve tumor-free mice or EL-4 tumor-bearing mice. CD11c+ cells were isolated from spleens of EL-4 tumor-bearing mice. Ten days later LN cells were isolated, re-stimulated with specific (S.P.) or control (C.P.) peptide and the number of IFN-γ producing cells was analyzed by ELISPOT assay. Results presented as Mean±SD. Two experiments with the same results were performed. B. OT-1 T cells were transferred i.v. into naïve C57BL/6 mice. Mice were immunized with SIINFEKL. CD11c+ cells were isolated from EL-4 tumor-bearing mice, pulsed with the specific peptide, and administered i.v. into mice 8 days after immunization. LN cells were collected 24 hr later and stimulated with either control or specific peptide. The number (Mean ± SD) of IFN-γ producing cells per 106 LN cells is shown. C. Gr-1+ cells isolated from tumor-bearing mice were transferred first into naïve recipient mice. Three days later these mice received OT-1 T cells and 2 days after that, mice were immunized with SIINFEKL peptide. LN cells were collected 10 days after immunization and were re-stimulated with control or specific peptides and the number of IFN-γ producing cells was analyzed in ELISPOT assay. Results presented as Mean±SD.
Figure 4. The mechanisms of CD8+ T-cell tolerance induction by ImC
A. Adoptive transfer of OT-1 cells, Gr-1+ cells and immunization with peptide was done as shown above in Fig.1A. LN cells were isolated on day 10 after immunization and percentage of antigen-specific CD8+Valpha2+ T cells was determined by flow cytometry. A representative experiment of three performed is shown. B. Adoptive transfer of OT-1 cells, Gr-1+ cells and immunization with peptide was done as shown in Fig.1A. Draining LNs were re-stimulated in vitro with specific (S.P.) or control (C.P.) peptides (10 μg/ml) or anti-CD3 antibody (1μg/ml) for 48 h. Production of IFN-γ was measured using intracellular staining within antigen-specific CD8+Vα2+ T cells. Typical results of one of two performed experiments are shown. C. 2C T cells (3 x 106) were adoptively transferred i.v. into naive C57BL/6 recipients followed by 3 days later immunization with 2C specific peptide SIYRYYGL (100 μg) and injection of Gr-1+ cells (3 x 106) from EL-4 tumor-bearing mice. DCs generated in vitro from control C57BL/6 mice were activated with LPS, pulsed with either specific (SIY) or control (QL9) peptides, and injected s.c. (4x105) into mice 10 days after first immunization. In 7 days after that mice were sacrificed, LN cells isolated and re-stimulated with specific (S.P.) or control (C.P.) peptides. The number of IFN-γ producing cells was measured by ELISPOT. Results presented as Mean±SD. D. T cells isolated from OT-1 mice were transferred i.v. into naïve C57BL/6 mice. Mice were immunized with SIINFEKL together with the transfer of Gr-1+ isolated from spleens of EL-4 tumor-bearing mice. Ten days later LN cells were collected and T cells were isolated and added in triplicates at indicated ratio to 105 splenocytes isolated from naïve mice and incubated for 4 days with 0.5 μg/ml anti-CD3 antibody and 0.1 μg/ml anti-CD28 antibody. Cell proliferation was evaluated using 3H-thymidine uptake. Results presented as Mean±SD. Control T cells – T cells isolated from immunized mice without transfer of Gr-1+ImC, tolerized T cells – T cells isolated from mice after adoptive transfer of Gr-1+ cells. E. Adoptive transfer of OT-1 cells, Gr-1+ cells and immunization with peptide was done as shown in Fig.1A. LN cells were isolated on day 9 after immunization and percentage of CD4+CD25+ cells was determined by flow cytometry. Typical results of one of two performed experiments are shown.
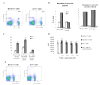
Figure 5. ImC ability to induce CD8+ T-cell tolerance in tumor-bearing mice
A. Cell detection in tumor tissues. Gr-1+ cells were labeled with 20 μM cell tracker orange 5-(and-6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine (CMTMR, Molecular Probes, Eugene, OR) and then injected (5x106/mouse) intravenously. Tumor tissues were taken out at different time points and snap-frozen in tissue freezing media (Triangle Biomedical Sciences, Durham, NC) at −80 °C. Frozen tissue sections were examined by fluorescence microscopy using a 540 nm filter. Labeled cells were counted in 10 fields at the total magnification x200. B. ImC take up soluble protein in vivo. Tumor-bearing mice were injected i.p. with 0.5 mg of OVA-FITC. Two hours later spleens were isolated and percentage of OVA-positive cells was determined within Gr-1+CD11b+ population of ImC. Typical results of one of two performed experiments are shown. C and D. C57BL/6 mice were inoculated s.c. with EG-7 or parent EL-4 tumor cells. Two weeks later when tumor reached 1.5 cm in diameter spleens and LNs were isolated and used in further experiments. B, C. Splenocytes and LN cells were stained with anti-Gr-1-APC and CD11b+-PE antibodies (Gr-1+), anti-CD11c-APC antibody (CD11c+), or anti-F4/80 antibody (F4/80+) in combination with anti-OVA 25-D1.16 antibody followed by staining with anti-mouse IgG-FITC. OVA-epitope positive cells were calculated after subtraction of the proportion of cells stained positive with isotype control IgG from cells stained positive with 25-D1.16 antibody. C. The result of typical experiment is shown (splenocytes). D. The proportion of OVA positive cells was calculated within each population of cells. Three experiments with similar results were performed. E. Recipients of OT-1 cells were immunized with SIINFEKL as described above. Gr-1+ cells were isolated from spleens of EL-4 or EG-7 tumor-bearing mice and these non-manipulated cells were injected into mice 8 days after immunization. As positive control Gr-1+ cells isolated from EL-4 tumor-bearing mice and pulsed with specific peptide were used. LN cells were collected 24 hr later and re-stimulated in triplicates with control (C.P.) or specific (S.P.) peptide. The number of IFN-γ producing cells was calculated per 106 LN cells.
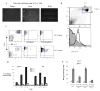
Figure 6. Effect of ImC on tumor growth
A. MethA sarcoma cells were inoculated s.c. (3x105 cells per mice) into 12 BALB/c mice. Tumor volume was monitored as indicated and calculated as follows: Tumor Volume= 0.5 (length) (breadth) (breadth). Five mice eventually rejected tumor (regressing MethA) and 7 had progressing tumor (progressing MethA). Tumor size for each group was traced back to day 6 after tumor inoculation. B. Splenocytes were labeled with anti-Gr-1-APC and anti-CD11b-PE antibodies and the percentage of double-positive cells was evaluated by flow cytometry. Background level of Gr-1+CD11b+ ImC was established in naïve tumor-free mice (designates as day 0). On day 6 after MethA sarcoma inoculation the presence of ImC was evaluated in 6 mice. All mice showed the same slightly elevated level of ImC. On day 12, mice were split into two groups (with progressing and regressing tumors). Three mice per group were evaluated. The same groups were analyzed on day 25. C and D. MethA sarcoma cells (C) or CT-26 tumor cells (D) (both 5x105 cells per mouse) were inoculated s.c. into BALB/c mice. Five days later mice were split into two groups (4 mice per group for each tumor model) with equal tumor volume. Control groups have received i.v. injection of 0.2 ml PBS, Gr-1 groups have received 4x106 Gr-1+ ImC derived either from CT-26 tumor-bearing mice (injected into MethA sarcoma-bearing mice) or from MethA tumor-bearing mice (injected into CT-26 tumor-bearing mice). Tumor volume was monitored as indicated. Mean ± SD are shown.
Similar articles
- Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species.
Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Kusmartsev S, et al. J Immunol. 2004 Jan 15;172(2):989-99. doi: 10.4049/jimmunol.172.2.989. J Immunol. 2004. PMID: 14707072 - T-cell-Secreted TNFα Induces Emergency Myelopoiesis and Myeloid-Derived Suppressor Cell Differentiation in Cancer.
Al Sayed MF, Amrein MA, Bührer ED, Huguenin AL, Radpour R, Riether C, Ochsenbein AF. Al Sayed MF, et al. Cancer Res. 2019 Jan 15;79(2):346-359. doi: 10.1158/0008-5472.CAN-17-3026. Epub 2018 Nov 2. Cancer Res. 2019. PMID: 30389698 - Mechanism of T cell tolerance induced by myeloid-derived suppressor cells.
Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Nagaraj S, et al. J Immunol. 2010 Mar 15;184(6):3106-16. doi: 10.4049/jimmunol.0902661. Epub 2010 Feb 8. J Immunol. 2010. PMID: 20142361 Free PMC article. - Analysis of splenic Gr-1int immature myeloid cells in tumor-bearing mice.
Yamamoto Y, Ishigaki H, Ishida H, Itoh Y, Noda Y, Ogasawara K. Yamamoto Y, et al. Microbiol Immunol. 2008 Jan;52(1):47-53. doi: 10.1111/j.1348-0421.2008.00009.x. Microbiol Immunol. 2008. PMID: 18352913 - Immature myeloid cells and cancer-associated immune suppression.
Kusmartsev S, Gabrilovich DI. Kusmartsev S, et al. Cancer Immunol Immunother. 2002 Aug;51(6):293-8. doi: 10.1007/s00262-002-0280-8. Epub 2002 Apr 24. Cancer Immunol Immunother. 2002. PMID: 12111117 Free PMC article. Review.
Cited by
- Improving the efficacy of immunotherapy for colorectal cancer: Targeting tumor microenvironment-associated immunosuppressive cells.
Zou D, Xin X, Xu Y, Xu H, Huang L, Xu T. Zou D, et al. Heliyon. 2024 Aug 16;10(16):e36446. doi: 10.1016/j.heliyon.2024.e36446. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39262952 Free PMC article. Review. - Tumor-associated CD8+T cell tolerance induced by erythroid progenitor cells.
Fan X, Peng H, Wang X, Sun Y, Dong Y, Zhou J, Chen J, Huang S. Fan X, et al. Front Immunol. 2024 May 10;15:1381919. doi: 10.3389/fimmu.2024.1381919. eCollection 2024. Front Immunol. 2024. PMID: 38799424 Free PMC article. - Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy.
Lu Q, Kou D, Lou S, Ashrafizadeh M, Aref AR, Canadas I, Tian Y, Niu X, Wang Y, Torabian P, Wang L, Sethi G, Tergaonkar V, Tay F, Yuan Z, Han P. Lu Q, et al. J Hematol Oncol. 2024 Apr 2;17(1):16. doi: 10.1186/s13045-024-01535-8. J Hematol Oncol. 2024. PMID: 38566199 Free PMC article. Review. - EP2 and EP4 blockade prevents tumor-induced suppressive features in human monocytic myeloid-derived suppressor cells.
Cuenca-Escalona J, Subtil B, Garcia-Perez A, Cambi A, de Vries IJM, Flórez-Grau G. Cuenca-Escalona J, et al. Front Immunol. 2024 Jan 26;15:1355769. doi: 10.3389/fimmu.2024.1355769. eCollection 2024. Front Immunol. 2024. PMID: 38343540 Free PMC article. - Construction and validation of a novel prognostic model of neutrophil‑related genes signature of lung adenocarcinoma.
Zhu Q, Chai Y, Jin L, Ma Y, Lu H, Chen Y, Feng W. Zhu Q, et al. Sci Rep. 2023 Oct 25;13(1):18226. doi: 10.1038/s41598-023-45289-8. Sci Rep. 2023. PMID: 37880277 Free PMC article.
References
- Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807. - PubMed
- Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780. - PubMed
- Cuenca A, Cheng F, Wang H, Brayer J, Horna P, Gu L, Bien H, Borrello IM, Levitsky HI, Sotomayor EM. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63:9007. - PubMed
- Bronte V, Serafini P, Appoloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunoth. 2001;24:431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials