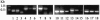Transcriptional profiling of target of RNAIII-activating protein, a master regulator of staphylococcal virulence - PubMed (original) (raw)
Transcriptional profiling of target of RNAIII-activating protein, a master regulator of staphylococcal virulence
Moshe Korem et al. Infect Immun. 2005 Oct.
Abstract
Staphylococcus aureus is a gram-positive bacterium that is part of the normal healthy flora but that can become virulent and cause infections by producing biofilms and toxins. The production of virulence factors is regulated by cell-cell communication (quorum sensing) through the histidine phosphorylation of target of RNAIII-activating protein (TRAP), which is a 21-kDa protein that is highly conserved among staphylococci. Using microarray analysis, we show here that the expression and phosphorylation of TRAP upregulate the expression of most, if not all, toxins known to date, as well as their global regulator agr. In addition, we show here that the expression and phosphorylation of TRAP are also necessary for the expression of genes known to be necessary for the survival of the bacteria in a biofilm, like arc, pyr, and ure. TRAP is thus demonstrated to be a master regulator of staphylococcal pathogenesis.
Figures
FIG. 1.
cDNA-PCR analysis of hld, spa, agrC, sspa, aur, and epbS. Specific primers (Table 1) were used to amplify the various genes using cDNA as a template (or DNA as a control). Lanes 1 to 3, rnaIII was amplified using DNA of 8325-4 (lane 1), cDNA of 8325-4 (lane 2), or cDNA of ΔTRAP (lane 3). Lanes 4 to 6, spa (encoding protein A) was amplified using DNA of 8325-4 (lane 4), cDNA of 8325-4 (lane 5), or cDNA of ΔTRAP (lane 6). Lanes 7 to 9, agrC (encoding accessory gene regulator C) was amplified using DNA of 8325-4 (lane 7), cDNA of 8325-4 (lane 8), or cDNA of ΔTRAP (lane 9). Lanes 10 to 12, sspA (encoding staphylococcal serine protease [V8 protease]) was amplified using DNA of 8325-4 (lane 10), cDNA of 8325-4 (lane 11), or cDNA of ΔTRAP (lane 12). Lanes 13 to 15, aur (encoding aureolysin) was amplified using DNA of 8325-4 (lane 13), cDNA of 8325-4 (lane 14), or cDNA of ΔTRAP (lane 15). Lanes 16 to 18, epbS (encoding elastin-binding protein) was amplified using DNA of 8325-4 (lane 16), cDNA of 8325-4 (lane 17), or cDNA of ΔTRAP (lane 18).
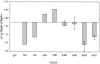
FIG. 2.
Real-time PCR quantification of the expression of indicated genes in postexponential S. aureus TRAP+ parent strain and its corresponding TRAP_-_ mutant. The values are an average of two to three replications normalized with respect to gyrB expression, and the data are expressed as the ratio of cycle (CT) threshold of TRAP-/TRAP+. Genes with a ratio is of >1 are downregulated in the TRAP-mutant.
FIG. 3.
Northern blot analysis of hla and hlb. Early exponential cells were grown from OD600 of 0.03 for 6 h (to the postexponential phase of growth). Cells were collected, Northern blotted, and membrane stained in methylene blue to visualize rRNA (top). mRNA of hla (α-hemolysin) and hlb (β-hemolysin) were detected with specific radiolabeled DNA. Membranes were autoradiographed. Lanes 1 to 2, hlb (β-hemolysin); lane 1, ΔTRAP; lane 2, 8325-4. Lanes 3 to 4, hla (α-hemolysin); lane 3, ΔTRAP; lane 4, 8325-4.
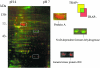
FIG. 4.
Two-dimensional gel electrophoresis of postexponential wild-type S. aureus 8325-4 (green) and the mutant strain ΔTRAP (red) using dual-channel images which were produced with Delta2D software (Decodon GmbH). Boxes indicate representative proteins that were differentially expressed.
Similar articles
- Inactivation of traP has no effect on the agr quorum-sensing system or virulence of Staphylococcus aureus.
Shaw LN, Jonsson IM, Singh VK, Tarkowski A, Stewart GC. Shaw LN, et al. Infect Immun. 2007 Sep;75(9):4519-27. doi: 10.1128/IAI.00491-07. Epub 2007 Jun 4. Infect Immun. 2007. PMID: 17548478 Free PMC article. - OpuC--an ABC transporter that is associated with Staphylococcus aureus pathogenesis.
Kiran MD, Akiyoshi DE, Giacometti A, Cirioni O, Scalise G, Balaban N. Kiran MD, et al. Int J Artif Organs. 2009 Sep;32(9):600-10. doi: 10.1177/039139880903200909. Int J Artif Organs. 2009. PMID: 19856269 - Quorum sensing-mediated regulation of staphylococcal virulence and antibiotic resistance.
Singh R, Ray P. Singh R, et al. Future Microbiol. 2014;9(5):669-81. doi: 10.2217/fmb.14.31. Future Microbiol. 2014. PMID: 24957093 Review. - TRAP plays a role in stress response in Staphylococcus aureus.
Kiran MD, Balaban N. Kiran MD, et al. Int J Artif Organs. 2009 Sep;32(9):592-9. doi: 10.1177/039139880903200908. Int J Artif Organs. 2009. PMID: 19856271 - Regulation of virulence determinants in Staphylococcus aureus.
Arvidson S, Tegmark K. Arvidson S, et al. Int J Med Microbiol. 2001 May;291(2):159-70. doi: 10.1078/1438-4221-00112. Int J Med Microbiol. 2001. PMID: 11437338 Review.
Cited by
- Methicillin-resistant Staphylococcus aureus as a cause of chronic wound infections: Alternative strategies for management.
Simonetti O, Marasca S, Candelora M, Rizzetto G, Radi G, Molinelli E, Brescini L, Cirioni O, Offidani A. Simonetti O, et al. AIMS Microbiol. 2022 Apr 24;8(2):125-137. doi: 10.3934/microbiol.2022011. eCollection 2022. AIMS Microbiol. 2022. PMID: 35974994 Free PMC article. Review. - Characterisation of Australian MRSA strains ST75- and ST883-MRSA-IV and analysis of their accessory gene regulator locus.
Monecke S, Kanig H, Rudolph W, Müller E, Coombs G, Hotzel H, Slickers P, Ehricht R. Monecke S, et al. PLoS One. 2010 Nov 17;5(11):e14025. doi: 10.1371/journal.pone.0014025. PLoS One. 2010. PMID: 21103340 Free PMC article. - Inactivation of traP has no effect on the agr quorum-sensing system or virulence of Staphylococcus aureus.
Shaw LN, Jonsson IM, Singh VK, Tarkowski A, Stewart GC. Shaw LN, et al. Infect Immun. 2007 Sep;75(9):4519-27. doi: 10.1128/IAI.00491-07. Epub 2007 Jun 4. Infect Immun. 2007. PMID: 17548478 Free PMC article. - Targeting Multidrug-resistant Staphylococci with an anti-rpoA Peptide Nucleic Acid Conjugated to the HIV-1 TAT Cell Penetrating Peptide.
Abushahba MF, Mohammad H, Seleem MN. Abushahba MF, et al. Mol Ther Nucleic Acids. 2016 Jul 19;5(7):e339. doi: 10.1038/mtna.2016.53. Mol Ther Nucleic Acids. 2016. PMID: 27434684 Free PMC article. - RNAIII-inhibiting peptide affects biofilm formation in a rat model of staphylococcal ureteral stent infection.
Cirioni O, Ghiselli R, Minardi D, Orlando F, Mocchegiani F, Silvestri C, Muzzonigro G, Saba V, Scalise G, Balaban N, Giacometti A. Cirioni O, et al. Antimicrob Agents Chemother. 2007 Dec;51(12):4518-20. doi: 10.1128/AAC.00808-07. Epub 2007 Sep 17. Antimicrob Agents Chemother. 2007. PMID: 17875996 Free PMC article.
References
- Balaban, N., A. Giacometti, O. Cirioni, Y. Gov, R. Ghiselli, F. Mocchegiani, C. Viticchi, M. S. Del Prete, V. Saba, G. Scalise, and G. Dell'Acqua. 2003. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 187:625-630. - PubMed
- Balaban, N., L. V. Collins, J. S. Cullor, E. B. Hume, E. Medina-Acosta, O. Vieira da Motta, R. O'Callaghan, P. V. Rossitto, M. E. Shirtliff, L. Serafim da Silveira, A. Tarkowski, and J. V. Torres. 2000. Prevention of diseases caused by Staphylococcus aureus using the peptide RIP. Peptides 21:1301-1311. - PubMed
- Balaban, N., P. Stoodley, C. A Fux, S. Wilson, J. W. Costerton, and G. Dell'Acqua. 2005. Prevention of staphylococcal biofilms-associated infections by the quorum sensing inhibitor RIP. Clin. Orthop. Relat. Res. 437:48-54. - PubMed
- Balaban, N., T. Goldkorn, R. T. Nhan, L. B. Dang, S. Scott, R. M. Ridgley, A. Rasooly, S. C. Wright, J. W. Larrick, R. Rasooly, and J. R. Carlson. 1998. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science 280:438-440. - PubMed
- Balaban, N., T. Goldkorn, Y. Gov, M. Hirshberg, N. Koyfman, H. R. Matthews, R. T. Nhan, B. Singh, and O. Uziel. 2001. Regulation of Staphylococcus aureus pathogenesis via target of RNAIII-activating protein (TRAP). J. Biol. Chem. 276:2658-2667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources