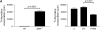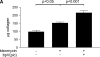Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) - PubMed (original) (raw)
Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10)
Eric S White et al. Am J Respir Crit Care Med. 2006.
Abstract
Rationale: Myofibroblasts are primary effector cells in idiopathic pulmonary fibrosis (IPF). Defining mechanisms of myofibroblast differentiation may be critical to the development of novel therapeutic agents.
Objective: To show that myofibroblast differentiation is regulated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) activity in vivo, and to identify a potential mechanism by which this occurs.
Methods: We used tissue sections of surgical lung biopsies from patients with IPF to localize expression of PTEN and alpha-smooth muscle actin (alpha-SMA). We used cell culture of pten(-/-) and wild-type fibroblasts, as well as adenoviral strategies and pharmacologic inhibitors, to determine the mechanism by which PTEN inhibits alpha-SMA, fibroblast proliferation, and collagen production.
Results: In human lung specimens of IPF, myofibroblasts within fibroblastic foci demonstrated diminished PTEN expression. Furthermore, inhibition of PTEN in mice worsened bleomycin-induced fibrosis. In pten(-/-) fibroblasts, and in normal fibroblasts in which PTEN was inhibited, alpha-SMA, proliferation, and collagen production was upregulated. Addition of transforming growth factor-beta to wild-type cells, but not pten(-/-) cells, resulted in increased alpha-SMA expression in a time-dependent fashion. In pten(-/-) cells, reconstitution of PTEN decreased alpha-SMA expression, proliferation, and collagen production, whereas overexpression of PTEN in wild-type cells inhibited transforming growth factor-beta-induced myofibroblast differentiation. It was observed that both the protein and lipid phosphatase actions of PTEN were capable of modulating the myofibroblast phenotype.
Conclusions: The results indicate that in IPF, myofibroblasts have diminished PTEN expression. Inhibition of PTEN in vivo promotes fibrosis, and PTEN inhibits myofibroblast differentiation in vitro.
Figures
**Figure 1.
(A) Immunohistochemical analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN; left panel) and α–smooth muscle actin (α-SMA; right panel) in a biopsy specimen from a patient with pulmonary fibrosis. Arrows identify clusters of spindle-shaped myofibroblasts (fibroblastic foci), which are characterized by α-SMA expression and a relative decrease in PTEN expression. Arrowheads delineate cuboidal epithelial cells (which express PTEN but not α-SMA) lining distorted airspaces. Photomicrographs are representative of fibroblastic foci observed throughout the lungs of all 10 patients examined (200× original magnification). (B) Triple immunofluorescent staining of the same biopsy specimen from the patient in (A) for PTEN (fluorescein isothiocyanate, green), α-SMA (Cy3, red), and nuclei (4′,6-diamidino-2-phenylindole [DAPI], blue). Whereas PTEN is located in epithelial and interstitial cells, it is not observed in α-SMA–expressing myofibroblasts (400× original magnification).
**Figure 1.
(A) Immunohistochemical analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN; left panel) and α–smooth muscle actin (α-SMA; right panel) in a biopsy specimen from a patient with pulmonary fibrosis. Arrows identify clusters of spindle-shaped myofibroblasts (fibroblastic foci), which are characterized by α-SMA expression and a relative decrease in PTEN expression. Arrowheads delineate cuboidal epithelial cells (which express PTEN but not α-SMA) lining distorted airspaces. Photomicrographs are representative of fibroblastic foci observed throughout the lungs of all 10 patients examined (200× original magnification). (B) Triple immunofluorescent staining of the same biopsy specimen from the patient in (A) for PTEN (fluorescein isothiocyanate, green), α-SMA (Cy3, red), and nuclei (4′,6-diamidino-2-phenylindole [DAPI], blue). Whereas PTEN is located in epithelial and interstitial cells, it is not observed in α-SMA–expressing myofibroblasts (400× original magnification).
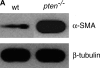
**Figure 2.
Loss of pten corresponds with increased α-SMA expression in fibroblasts. (A) Western blot of whole-cell lysates derived from serum-starved wild-type murine embryonic fibroblasts (wt) or embryonic fibroblasts from _pten_−/− mice (_pten_−/−) probed for α-SMA. Blots were stripped and reprobed for β-tubulin as a loading control. The results are representative of three independent experiments using three separate cultures of cells. (B) Indirect immunofluorescent analysis of serum-starved wt fibroblasts (left panel) or _pten_−/− fibroblasts (right panel) labeled with anti–α-SMA antibody. Results are representative of two independent experiments. (C) The wt fibroblasts were growth-arrested and then treated with either serum-free media (SF) or 200 nM bisperoxo(pyridine-2-carboxyl)oxovanadate (bpV[pic]) for 24 h, lysed, and immunoblotted for α-SMA, phosphorylated Akt at serine-473 (S473 pAkt), and total Akt. Results are representative of three separate experiments.
**Figure 2.
Loss of pten corresponds with increased α-SMA expression in fibroblasts. (A) Western blot of whole-cell lysates derived from serum-starved wild-type murine embryonic fibroblasts (wt) or embryonic fibroblasts from _pten_−/− mice (_pten_−/−) probed for α-SMA. Blots were stripped and reprobed for β-tubulin as a loading control. The results are representative of three independent experiments using three separate cultures of cells. (B) Indirect immunofluorescent analysis of serum-starved wt fibroblasts (left panel) or _pten_−/− fibroblasts (right panel) labeled with anti–α-SMA antibody. Results are representative of two independent experiments. (C) The wt fibroblasts were growth-arrested and then treated with either serum-free media (SF) or 200 nM bisperoxo(pyridine-2-carboxyl)oxovanadate (bpV[pic]) for 24 h, lysed, and immunoblotted for α-SMA, phosphorylated Akt at serine-473 (S473 pAkt), and total Akt. Results are representative of three separate experiments.
**Figure 2.
Loss of pten corresponds with increased α-SMA expression in fibroblasts. (A) Western blot of whole-cell lysates derived from serum-starved wild-type murine embryonic fibroblasts (wt) or embryonic fibroblasts from _pten_−/− mice (_pten_−/−) probed for α-SMA. Blots were stripped and reprobed for β-tubulin as a loading control. The results are representative of three independent experiments using three separate cultures of cells. (B) Indirect immunofluorescent analysis of serum-starved wt fibroblasts (left panel) or _pten_−/− fibroblasts (right panel) labeled with anti–α-SMA antibody. Results are representative of two independent experiments. (C) The wt fibroblasts were growth-arrested and then treated with either serum-free media (SF) or 200 nM bisperoxo(pyridine-2-carboxyl)oxovanadate (bpV[pic]) for 24 h, lysed, and immunoblotted for α-SMA, phosphorylated Akt at serine-473 (S473 pAkt), and total Akt. Results are representative of three separate experiments.
**Figure 3.
Reconstitution of PTEN into _pten_−/− cells inhibits the myofibroblast phenotype. (A, left panel) The wt and _pten_−/− cells were seeded in 96-well plates and allowed to proliferate in the presence of 3H-thymidine for 24 h. 3H-thymidine incorporation was measured by scintillation counting. Right panel: After infection with adenovirus encoding full-length, active PTEN (PTEN), but not empty virus (EV), proliferation was significantly attenuated. (B, left panel) Whole-cell lysates from wt and _pten_−/− cells were serum-starved for 24 h before evaluation for collagen production by Sircol assay. Right panel: The _pten_−/− cells were untreated (U) or infected with EV or active PTEN (Ad-PTEN, PTEN) for 24 h before lysis and evaluation of collagen production by Sircol assay. (C) The _pten_−/− cells were untreated (U), or treated with EV or with Ad-PTEN (PTEN) for 24 h. Lysates were assessed for α-SMA expression by Western blot. Blots were stripped and probed for β-tubulin as a loading control. Subsequently, the blot was stripped and reprobed to confirm PTEN expression.
**Figure 3.
Reconstitution of PTEN into _pten_−/− cells inhibits the myofibroblast phenotype. (A, left panel) The wt and _pten_−/− cells were seeded in 96-well plates and allowed to proliferate in the presence of 3H-thymidine for 24 h. 3H-thymidine incorporation was measured by scintillation counting. Right panel: After infection with adenovirus encoding full-length, active PTEN (PTEN), but not empty virus (EV), proliferation was significantly attenuated. (B, left panel) Whole-cell lysates from wt and _pten_−/− cells were serum-starved for 24 h before evaluation for collagen production by Sircol assay. Right panel: The _pten_−/− cells were untreated (U) or infected with EV or active PTEN (Ad-PTEN, PTEN) for 24 h before lysis and evaluation of collagen production by Sircol assay. (C) The _pten_−/− cells were untreated (U), or treated with EV or with Ad-PTEN (PTEN) for 24 h. Lysates were assessed for α-SMA expression by Western blot. Blots were stripped and probed for β-tubulin as a loading control. Subsequently, the blot was stripped and reprobed to confirm PTEN expression.
**Figure 3.
Reconstitution of PTEN into _pten_−/− cells inhibits the myofibroblast phenotype. (A, left panel) The wt and _pten_−/− cells were seeded in 96-well plates and allowed to proliferate in the presence of 3H-thymidine for 24 h. 3H-thymidine incorporation was measured by scintillation counting. Right panel: After infection with adenovirus encoding full-length, active PTEN (PTEN), but not empty virus (EV), proliferation was significantly attenuated. (B, left panel) Whole-cell lysates from wt and _pten_−/− cells were serum-starved for 24 h before evaluation for collagen production by Sircol assay. Right panel: The _pten_−/− cells were untreated (U) or infected with EV or active PTEN (Ad-PTEN, PTEN) for 24 h before lysis and evaluation of collagen production by Sircol assay. (C) The _pten_−/− cells were untreated (U), or treated with EV or with Ad-PTEN (PTEN) for 24 h. Lysates were assessed for α-SMA expression by Western blot. Blots were stripped and probed for β-tubulin as a loading control. Subsequently, the blot was stripped and reprobed to confirm PTEN expression.
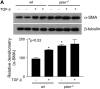
**Figure 4.
α-SMA expression in _pten_−/− cells is not dependent on autocrine TGF-β signaling or Smad activation. (A) The wt or _pten_−/− cells were cultured in serum-free media in the presence or absence of neutralizing antibody to TGF-β or control antibody for 24 h, and cell lysates were harvested for Western blot analysis of α-SMA. The blot was stripped and reprobed for β-tubulin as a loading control. Results are representative of two separate experiments. (B, top panel) The wt or _pten_−/− cells were cultured in serum-free media (SF) in the presence or absence of TGF-β (2 ng/ml) for 1 h, lysed, and assessed for phospho-Smad2 by Western blot. The membrane was stripped and reprobed with an antibody against total Smad2 to confirm equal loading. Results are representative of three separate experiments. Bottom panel: Lysates from serum-starved wt and _pten_−/− cells were collected and immunoblotted for total Smad7. The blot was stripped and reprobed for β-tubulin. Numbers under each lane represent densitometric ratios of Smad7 to β-tubulin.
**Figure 4.
α-SMA expression in _pten_−/− cells is not dependent on autocrine TGF-β signaling or Smad activation. (A) The wt or _pten_−/− cells were cultured in serum-free media in the presence or absence of neutralizing antibody to TGF-β or control antibody for 24 h, and cell lysates were harvested for Western blot analysis of α-SMA. The blot was stripped and reprobed for β-tubulin as a loading control. Results are representative of two separate experiments. (B, top panel) The wt or _pten_−/− cells were cultured in serum-free media (SF) in the presence or absence of TGF-β (2 ng/ml) for 1 h, lysed, and assessed for phospho-Smad2 by Western blot. The membrane was stripped and reprobed with an antibody against total Smad2 to confirm equal loading. Results are representative of three separate experiments. Bottom panel: Lysates from serum-starved wt and _pten_−/− cells were collected and immunoblotted for total Smad7. The blot was stripped and reprobed for β-tubulin. Numbers under each lane represent densitometric ratios of Smad7 to β-tubulin.
**Figure 4.
α-SMA expression in _pten_−/− cells is not dependent on autocrine TGF-β signaling or Smad activation. (A) The wt or _pten_−/− cells were cultured in serum-free media in the presence or absence of neutralizing antibody to TGF-β or control antibody for 24 h, and cell lysates were harvested for Western blot analysis of α-SMA. The blot was stripped and reprobed for β-tubulin as a loading control. Results are representative of two separate experiments. (B, top panel) The wt or _pten_−/− cells were cultured in serum-free media (SF) in the presence or absence of TGF-β (2 ng/ml) for 1 h, lysed, and assessed for phospho-Smad2 by Western blot. The membrane was stripped and reprobed with an antibody against total Smad2 to confirm equal loading. Results are representative of three separate experiments. Bottom panel: Lysates from serum-starved wt and _pten_−/− cells were collected and immunoblotted for total Smad7. The blot was stripped and reprobed for β-tubulin. Numbers under each lane represent densitometric ratios of Smad7 to β-tubulin.
**Figure 5.
Increased basal expression of α-SMA in _pten_−/− cells is due to increased gene expression and transcription. (A) Two separate aliquots of wt and _pten_−/− cells were cultured in serum-free media in the presence or absence of TGF-β (2 ng/ml) for 24 h. Whole-cell lysates were evaluated by Western blot for α-SMA expression. To verify equal protein loading, the membrane was stripped and reprobed for β-tubulin. (B) The wt and _pten_−/− cells were cultured in serum-free media for 24 h and RNA was isolated. α-SMA gene expression was evaluated by semiquantitative real-time PCR using glyceraldehyde phosphate dehydrogenase as an internal control. Results are pooled data from two separate experiments performed in triplicate.
**Figure 5.
Increased basal expression of α-SMA in _pten_−/− cells is due to increased gene expression and transcription. (A) Two separate aliquots of wt and _pten_−/− cells were cultured in serum-free media in the presence or absence of TGF-β (2 ng/ml) for 24 h. Whole-cell lysates were evaluated by Western blot for α-SMA expression. To verify equal protein loading, the membrane was stripped and reprobed for β-tubulin. (B) The wt and _pten_−/− cells were cultured in serum-free media for 24 h and RNA was isolated. α-SMA gene expression was evaluated by semiquantitative real-time PCR using glyceraldehyde phosphate dehydrogenase as an internal control. Results are pooled data from two separate experiments performed in triplicate.
**Figure 6.
Inhibition of PTEN is necessary for TGF-β–induced α-SMA expression. Wt, serum-starved fibroblasts were induced to express α-SMA with TGF-β (2 ng/ml) in the presence or absence of an adenovirus encoding full-length Ad-PTEN or empty virus alone (Ad-EV). Whole-cell lysates were assessed for α-SMA expression by Western blot. The same blot was sequentially stripped and reprobed for PTEN and β-tubulin. The results are representative of two independent experiments.
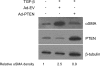
**Figure 7.
PTEN suppression of α-SMA expression involves both lipid- and protein-phosphatase activity. (A) Wt, serum-starved fibroblasts were induced to express α-SMA by treating with TGF-β (2 ng/ml) or bpV(pic) (200 nM) in the presence or absence of PP2 (10 μM) or PP3 (10 μM) for 24 h. Whole-cell lysates were prepared and evaluated by Western blot for α-SMA. The blot was stripped and reprobed for β-tubulin to confirm equal protein loading. The blot is representative of two separate experiments. (B) Wt, serum-starved fibroblasts were induced to express α-SMA by treating with TGF-β (2 ng/ml) or bpV(pic) (200 nM) in the presence or absence of the PI3K inhibitors LY294002 (50 μM) or wortmannin (50 nM) for 24 h. Whole-cell lysates were prepared and evaluated by Western blot for α-SMA. The blot was stripped and reprobed for β-tubulin to confirm equal protein loading. The blot is representative of two separate experiments.
**Figure 7.
PTEN suppression of α-SMA expression involves both lipid- and protein-phosphatase activity. (A) Wt, serum-starved fibroblasts were induced to express α-SMA by treating with TGF-β (2 ng/ml) or bpV(pic) (200 nM) in the presence or absence of PP2 (10 μM) or PP3 (10 μM) for 24 h. Whole-cell lysates were prepared and evaluated by Western blot for α-SMA. The blot was stripped and reprobed for β-tubulin to confirm equal protein loading. The blot is representative of two separate experiments. (B) Wt, serum-starved fibroblasts were induced to express α-SMA by treating with TGF-β (2 ng/ml) or bpV(pic) (200 nM) in the presence or absence of the PI3K inhibitors LY294002 (50 μM) or wortmannin (50 nM) for 24 h. Whole-cell lysates were prepared and evaluated by Western blot for α-SMA. The blot was stripped and reprobed for β-tubulin to confirm equal protein loading. The blot is representative of two separate experiments.
**Figure 7.
PTEN suppression of α-SMA expression involves both lipid- and protein-phosphatase activity. (A) Wt, serum-starved fibroblasts were induced to express α-SMA by treating with TGF-β (2 ng/ml) or bpV(pic) (200 nM) in the presence or absence of PP2 (10 μM) or PP3 (10 μM) for 24 h. Whole-cell lysates were prepared and evaluated by Western blot for α-SMA. The blot was stripped and reprobed for β-tubulin to confirm equal protein loading. The blot is representative of two separate experiments. (B) Wt, serum-starved fibroblasts were induced to express α-SMA by treating with TGF-β (2 ng/ml) or bpV(pic) (200 nM) in the presence or absence of the PI3K inhibitors LY294002 (50 μM) or wortmannin (50 nM) for 24 h. Whole-cell lysates were prepared and evaluated by Western blot for α-SMA. The blot was stripped and reprobed for β-tubulin to confirm equal protein loading. The blot is representative of two separate experiments.
**Figure 8.
Inhibition of PTEN worsens experimental fibrosis. (A) C57Bl/6 mice administered intratracheal bleomycin were treated in the presence or absence of the PTEN inhibitor bpV(pic). Animals were killed 21 d after bleomycin treatment, and lungs were assessed for total collagen. Mice treated with intratracheal saline were used as controls (n = 8 for each experimental group). The figure is representative of two separately performed experiments. (B) a and b: Lung sections from a mouse receiving intratracheal saline. c and d: Lung sections from a mouse receiving intratracheal bleomycin. e and f: Lung sections from a mouse receiving intratracheal bleomycin and intraperitoneal bpV(pic). Left panels (a, c, e): Trichrome stain. Right panels (b, d, f): Immunofluorescent stain for α-SMA (red) and nuclear DAPI stain (blue). Arrows are pointing to individual cells or clusters of myofibroblasts staining positively for α-SMA. Arrowheads identify α-SMA–expressing smooth muscle cells lining large airways. α-SMA–expressing myofibroblasts colocalize to fibrotic regions of the lung (200× original magnification).
**Figure 8.
Inhibition of PTEN worsens experimental fibrosis. (A) C57Bl/6 mice administered intratracheal bleomycin were treated in the presence or absence of the PTEN inhibitor bpV(pic). Animals were killed 21 d after bleomycin treatment, and lungs were assessed for total collagen. Mice treated with intratracheal saline were used as controls (n = 8 for each experimental group). The figure is representative of two separately performed experiments. (B) a and b: Lung sections from a mouse receiving intratracheal saline. c and d: Lung sections from a mouse receiving intratracheal bleomycin. e and f: Lung sections from a mouse receiving intratracheal bleomycin and intraperitoneal bpV(pic). Left panels (a, c, e): Trichrome stain. Right panels (b, d, f): Immunofluorescent stain for α-SMA (red) and nuclear DAPI stain (blue). Arrows are pointing to individual cells or clusters of myofibroblasts staining positively for α-SMA. Arrowheads identify α-SMA–expressing smooth muscle cells lining large airways. α-SMA–expressing myofibroblasts colocalize to fibrotic regions of the lung (200× original magnification).
Comment in
- PTEN as a new agent in the fight against fibrogenesis.
Kuwano K. Kuwano K. Am J Respir Crit Care Med. 2006 Jan 1;173(1):5-6. doi: 10.1164/rccm.2510001. Am J Respir Crit Care Med. 2006. PMID: 16368792 No abstract available.
Similar articles
- Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration.
Fagone E, Conte E, Gili E, Fruciano M, Pistorio MP, Lo Furno D, Giuffrida R, Crimi N, Vancheri C. Fagone E, et al. Exp Lung Res. 2011 Apr;37(3):162-74. doi: 10.3109/01902148.2010.524722. Epub 2011 Jan 26. Exp Lung Res. 2011. PMID: 21269063 - Heat Shock Protein 27 Plays a Pivotal Role in Myofibroblast Differentiation and in the Development of Bleomycin-Induced Pulmonary Fibrosis.
Park AM, Kanai K, Itoh T, Sato T, Tsukui T, Inagaki Y, Selman M, Matsushima K, Yoshie O. Park AM, et al. PLoS One. 2016 Feb 9;11(2):e0148998. doi: 10.1371/journal.pone.0148998. eCollection 2016. PLoS One. 2016. PMID: 26859835 Free PMC article. - Aortic carboxypeptidase-like protein (ACLP) enhances lung myofibroblast differentiation through transforming growth factor β receptor-dependent and -independent pathways.
Tumelty KE, Smith BD, Nugent MA, Layne MD. Tumelty KE, et al. J Biol Chem. 2014 Jan 31;289(5):2526-36. doi: 10.1074/jbc.M113.502617. Epub 2013 Dec 16. J Biol Chem. 2014. PMID: 24344132 Free PMC article. - Molecular targets in pulmonary fibrosis: the myofibroblast in focus.
Scotton CJ, Chambers RC. Scotton CJ, et al. Chest. 2007 Oct;132(4):1311-21. doi: 10.1378/chest.06-2568. Chest. 2007. PMID: 17934117 Review. - The myofibroblast in pulmonary fibrosis.
Phan SH. Phan SH. Chest. 2002 Dec;122(6 Suppl):286S-289S. doi: 10.1378/chest.122.6_suppl.286s. Chest. 2002. PMID: 12475801 Review.
Cited by
- Pulmonary hypertension secondary to left-heart failure involves peroxynitrite-induced downregulation of PTEN in the lung.
Ravi Y, Selvendiran K, Naidu SK, Meduru S, Citro LA, Bognár B, Khan M, Kálai T, Hideg K, Kuppusamy P, Sai-Sudhakar CB. Ravi Y, et al. Hypertension. 2013 Mar;61(3):593-601. doi: 10.1161/HYPERTENSIONAHA.111.00514. Epub 2013 Jan 21. Hypertension. 2013. PMID: 23339168 Free PMC article. - Developing PI3K Inhibitors for Respiratory Diseases.
Fagone E, Fruciano M, Gili E, Sambataro G, Vancheri C. Fagone E, et al. Curr Top Microbiol Immunol. 2022;436:437-466. doi: 10.1007/978-3-031-06566-8_19. Curr Top Microbiol Immunol. 2022. PMID: 36243856 - Sphingolipid regulation of tissue fibrosis.
Shea BS, Tager AM. Shea BS, et al. Open Rheumatol J. 2012;6:123-9. doi: 10.2174/1874312901206010123. Epub 2012 Jun 15. Open Rheumatol J. 2012. PMID: 22802910 Free PMC article. - Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF.
Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, Franklin L, Nanthakumar CB, Man Y, Genovese F, McAnulty RJ, Yang S, Maher TM, Nicholson AG, Blanchard AD, Marshall RP, Lukey PT, Chambers RC. Mercer PF, et al. Thorax. 2016 Aug;71(8):701-11. doi: 10.1136/thoraxjnl-2015-207429. Epub 2016 Apr 21. Thorax. 2016. PMID: 27103349 Free PMC article. - Altered DNA methylation profile in idiopathic pulmonary fibrosis.
Sanders YY, Ambalavanan N, Halloran B, Zhang X, Liu H, Crossman DK, Bray M, Zhang K, Thannickal VJ, Hagood JS. Sanders YY, et al. Am J Respir Crit Care Med. 2012 Sep 15;186(6):525-35. doi: 10.1164/rccm.201201-0077OC. Epub 2012 Jun 14. Am J Respir Crit Care Med. 2012. PMID: 22700861 Free PMC article.
References
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971;27:549–550. - PubMed
- Zhang K, Gharaee-Kermani M, McGarry B, Phan SH. In situ hybridization analysis of rat lung alpha 1(I) and alpha 2(I) collagen gene expression in pulmonary fibrosis induced by endotracheal bleomycin injection. Lab Invest 1994;70:192–202. - PubMed
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4, 5-trisphosphate. J Biol Chem 1998;273:13375–13378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL56402/HL/NHLBI NIH HHS/United States
- HL070990/HL/NHLBI NIH HHS/United States
- HL52285/HL/NHLBI NIH HHS/United States
- L30 HL074723/HL/NHLBI NIH HHS/United States
- K08 HL070990/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials