Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness - PubMed (original) (raw)
Review
Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness
Brent A Vogt et al. Prog Brain Res. 2005.
Abstract
Neuronal aggregates involved in conscious awareness are not evenly distributed throughout the CNS but comprise key components referred to as the neural network correlates of consciousness (NNCC). A critical node in this network is the posterior cingulate, precuneal, and retrosplenial cortices. The cytological and neurochemical composition of this region is reviewed in relation to the Brodmann map. This region has the highest level of cortical glucose metabolism and cytochrome c oxidase activity. Monkey studies suggest that the anterior thalamic projection likely drives retrosplenial and posterior cingulate cortex metabolism and that the midbrain projection to the anteroventral thalamic nucleus is a key coupling site between the brainstem system for arousal and cortical systems for cognitive processing and awareness. The pivotal role of the posterior cingulate, precuneal, and retrosplenial cortices in consciousness is demonstrated with posterior cingulate epilepsy cases, midcingulate lesions that de-afferent this region and are associated with unilateral sensory neglect, observations from stroke and vegetative state patients, alterations in blood flow during sleep, and the actions of general anesthetics. Since this region is critically involved in self reflection, it is not surprising that it is similarly a site for the NNCC. Interestingly, information processing during complex cognitive tasks and during aversive sensations such as pain induces efforts to terminate self reflection and result in decreased processing in posterior cingulate and precuneal cortices.
Figures
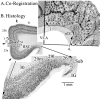
Figure 1
A. Co-registration of Brodmann’s map of posterior cingulate and precuneal cortices and a postmortem case for joint histological assessment. Stroked dots outline the border of cingulate cortex and four numbered arrows refer to locations of particular Brodmann areas discussed in the text. Brodmann’s actual numbers 23 and 31 are shown in the co-registration, while all other numbers refer to Vogt et al. (2005). The arrow at 4. points to the level of the coronal histological section taken for B. A critical issue at 4. is the manner in which Brodmann extended retrosplenial areas 29 and 30 onto the gyral surface where area 23 is located. RSC comprises the ventral bank of the cingulate gyrus in the callosal sulcus and is not exposed as suggested in his map. Abbreviations: cgs, cingulate sulcus; IG, indusium griseum; mr, marginal ramus of the cgs; pos, parieto-occipital sulcus; sCC, splenium of the corpus callosum; Sub, dorsal subiculum; VCA, vertical plane at the anterior commissure;
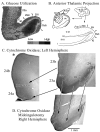
Figure 2
Morphological context of metabolic activity in PCC in monkeys. A. 2-deoxy-D-glucose utilization coded for four levels of utilization and thalamic projections to RSC shown with a tritiated-amino acid injection (hatched) into the anterior thalamic nuclei and a coronal section through RSC areas 29 and 30. The close relationship between high glucose metabolism and thalamic afferents are obvious. Interestingly, high levels of the mitochondrial enzyme cytochrome c oxidase also occur in the granular layer of RSC and in layers III-IV of areas 30 and 23. The asterisk in B shows where the section through ACC in C. was taken. Notice that ACC has much less cytochrome c oxidase activity than does area 23 (shown with the pair of arrows delineating these areas). A midcingulotomy lesion (D.; at coronal level shown with “v” on medial surface in B.) that removes thalamic afferents to PCC/RSC as well as frontal lobe inputs shows a massive reduction of activity in the thalamoreceptive layers as predicted from selective thalamic lesions in rat. There is about a 20% volumetric reduction in the posterior cingulate gyrus and reductions in enzyme activity are emphasized with three arrows from layer III/IV in area 29 and layers III and IV in areas 30 and 23c. Thus, high metabolic activity in PCC, RSC, and PrCC is driven primarily by thalamic afferents.
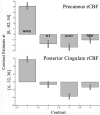
Figure 3
Estimates of rCBF in precuneal and posterior cingulate cortices during wakefulness and three stages of sleep. The pattern is similar for both cortices with a high level of rCBF during wakefulness and reductions during sleep. The one main difference between these areas is that PCC during stage II sleep has a higher level of activity than during REM than is the case for PrCC. Thus, the transition to sleep involves greater reductions in rCBF in PrCC than in PrCC.
Similar articles
- Macaque monkey retrosplenial cortex: II. Cortical afferents.
Kobayashi Y, Amaral DG. Kobayashi Y, et al. J Comp Neurol. 2003 Nov 3;466(1):48-79. doi: 10.1002/cne.10883. J Comp Neurol. 2003. PMID: 14515240 - Cytology and functionally correlated circuits of human posterior cingulate areas.
Vogt BA, Vogt L, Laureys S. Vogt BA, et al. Neuroimage. 2006 Jan 15;29(2):452-66. doi: 10.1016/j.neuroimage.2005.07.048. Epub 2005 Sep 6. Neuroimage. 2006. PMID: 16140550 Free PMC article. Clinical Trial. - [Cingulate gyrus: cortical architecture and connections].
Kobayashi Y. Kobayashi Y. Brain Nerve. 2011 May;63(5):473-82. Brain Nerve. 2011. PMID: 21515927 Review. Japanese. - Consciousness supporting networks.
Demertzi A, Soddu A, Laureys S. Demertzi A, et al. Curr Opin Neurobiol. 2013 Apr;23(2):239-44. doi: 10.1016/j.conb.2012.12.003. Epub 2012 Dec 27. Curr Opin Neurobiol. 2013. PMID: 23273731 Review. - Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex.
Lavenex P, Suzuki WA, Amaral DG. Lavenex P, et al. J Comp Neurol. 2002 Jun 10;447(4):394-420. doi: 10.1002/cne.10243. J Comp Neurol. 2002. PMID: 11992524
Cited by
- Abnormal prediction error processing in schizophrenia and depression.
Yaple ZA, Tolomeo S, Yu R. Yaple ZA, et al. Hum Brain Mapp. 2021 Aug 1;42(11):3547-3560. doi: 10.1002/hbm.25453. Epub 2021 May 6. Hum Brain Mapp. 2021. PMID: 33955106 Free PMC article. - Evaluation of temporomandibular joint, masticatory muscle, and brain cortex activity in patients treated by removable functional appliances: a prospective fMRI study.
Ozdiler O, Orhan K, Cesur E, Köklü A, Algın O. Ozdiler O, et al. Dentomaxillofac Radiol. 2019 Oct;48(7):20190216. doi: 10.1259/dmfr.20190216. Epub 2019 Jul 24. Dentomaxillofac Radiol. 2019. PMID: 31322927 Free PMC article. - Topographical relocation of adolescent sleep spindles reveals a new maturational pattern in the human brain.
Gombos F, Bódizs R, Pótári A, Bocskai G, Berencsi A, Szakács H, Kovács I. Gombos F, et al. Sci Rep. 2022 Apr 29;12(1):7023. doi: 10.1038/s41598-022-11098-8. Sci Rep. 2022. PMID: 35487959 Free PMC article. - Imaging the functional connectivity of the Periaqueductal Gray during genuine and sham electroacupuncture treatment.
Zyloney CE, Jensen K, Polich G, Loiotile RE, Cheetham A, LaViolette PS, Tu P, Kaptchuk TJ, Gollub RL, Kong J. Zyloney CE, et al. Mol Pain. 2010 Nov 16;6:80. doi: 10.1186/1744-8069-6-80. Mol Pain. 2010. PMID: 21080967 Free PMC article. Clinical Trial. - Nonacceptance of negative emotions in women with borderline personality disorder: association with neuroactivity of the dorsal striatum.
Lamers A, Toepper M, Fernando SC, Schlosser N, Bauer E, Woermann F, Driessen M, Beblo T. Lamers A, et al. J Psychiatry Neurosci. 2019 Sep 1;44(5):303-312. doi: 10.1503/jpn.180077. J Psychiatry Neurosci. 2019. PMID: 30964611 Free PMC article.
References
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–1338. - PubMed
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg. 1997;84:120–126. - PubMed
- Alkaire MT, Pomfrett MT, Haier CJ, Gianzero RJ, Chan MV, Jacobson BP, Fallon JH. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology. 1999;90:701–709. - PubMed
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: Two facts of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. - PubMed
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of posterior cingulate cortex during generalized spike and wave. NeuroImage. 2003;20:1915–1922. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources