Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation - PubMed (original) (raw)
Comparative Study
. 2005 Oct 3;202(7):919-29.
doi: 10.1084/jem.20050463. Epub 2005 Sep 26.
Affiliations
- PMID: 16186184
- PMCID: PMC2213166
- DOI: 10.1084/jem.20050463
Comparative Study
Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation
François Ghiringhelli et al. J Exp Med. 2005.
Abstract
The mechanisms through which regulatory T cells accumulate in lymphoid organs of tumor-bearing hosts remain elusive. Our experiments indicate that the accumulation of CD4+CD25+ regulatory T cells (T reg cells) expressing FoxP3 and exhibiting immunosuppressive function originates from the proliferation of naturally occurring CD25+ T cells and requires signaling through transforming growth factor (TGF)-beta receptor II. During tumor progression, a subset of dendritic cells (DCs) exhibiting a myeloid immature phenotype is recruited to draining lymph nodes. This DC subset selectively promotes the proliferation of T reg cells in a TGF-beta-dependent manner in mice and rats. Tumor cells are necessary and sufficient to convert DCs into regulatory cells that secrete bioactive TGF-beta and stimulate T reg cell proliferation. In conclusion, tumor expansion can stimulate T reg cells via a specific DC subset.
Figures
Figure 1.
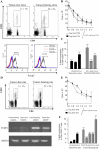
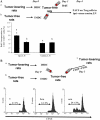
Accumulation and proliferation of T reg cells in lymphoid organs of tumor-bearing rodents. (A) CD4+CD25+ cells in DLNs from C57BL6 mice bearing B16F10 tumors. The expression of CD4 and CD25 was analyzed by flow cytometry on gated CD3+ T cells isolated from inguinal DLNs of 15-d-old B16F10 TBM or tumor-free controls. These cells were FACS purified and then subjected to intracellular staining for the detection of FOXP3, whereas background levels were determined with an isotype control (bottom). Percent values represent the mean ± SEM (n = 6 distinct animals). (B) Inhibitory effect of T reg cell splenocytes. 105 splenocytes harvested from a TFM were depleted in CD25+ cells and stimulated with 2 μg/ml of ConA. CD25+ T cells purified from TFM or TBM were added to the culture. Proliferation was determined after 3 d of culture using [3H]thymidine incorporation. Values represent the mean ± SEM of triplicates. (C) Proliferation of T reg cells in TBM. C57BL6 mice bearing B16F10 tumors (as in A) or TFM were injected with BrdU, and the frequency of BrdU+ cells was determined among CD3+CD4+CD25+ cells by flow cytometry. Values represent the mean ± SEM (n = 6). (D) Immunophenotyping of axillary DLNs from rats bearing 28-d-old PROb tumors or tumor free control LNs. Percent values represent the mean ± SEM (n = 6). RT-PCR analysis of FOXP3 expression in CD25+ and CD25− T cells from TBR and TFR. Data are representative of three experiments. (E) Antiproliferative action of T reg cells from rats. The experiment was analogous to that shown in B, with the difference that all cells were derived from BD-IX rats instead of C57BL/6 mice. (F) Proliferation of T reg cells in vivo in TBR. BD-IX rats bearing PROb tumors (as in D) or TFR were injected with BrdU, and the frequency of BrdU+ cells was determined among CD3+CD4+CD25+ cells as in C. Values represent the mean ± SEM (n = 6).
Figure 2.
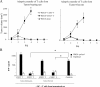
Adoptive transfer of T reg cells into immunogenic tumor variants. (A) Adoptive transfer of T reg cells into rats bearing immunogenic REGb tumors promotes tumor outgrowth. 106 REGb cells were inoculated s.c. into the flank, and tumor kinetics were monitored. REGb cells were either injected alone or admixed with either 10 × 106 CD25+ or CD25− T cells derived from TFR or rats bearing 42-d-old PROb tumors. The results of a representative experiment containing five rats per group is shown, and statistical analyses were performed using Fisher's exact test at 95% confidence. Values represent the mean ± SEM. (B) CD25+ T cells abrogate antigen-driven IFN-γ secretion from effector T cells. 105 splenic T cells isolated from rats immunized against PROb cells were stimulated with 104 CD11c+ DCs purified from naive animals and admixed with lysates from 104 PROb cells. CD25−/+ T cells from tumor-bearing or naive animals were added to the DC/T cell co-culture. IFN-γ secretion in the culture supernatant was determined by ELISA after 48 h of co-culture. Values represent the mean ± SEM (n = 3). Asterisks indicate significant differences at a P < 0.05 confidence interval using Fisher's exact test.
Figure 3.
Depletion of T reg cells in nonimmunogenic tumors. (A) Depletion of CD25+ T reg cells promotes tumor regression in the PROb model. Rats bearing 21-d-old s.c. PROb tumors were injected with either 1 mg of anti–rat CD25 antibody (Clone OX-39; closed square) or control isotype (open diamond). The results of a representative experiment containing five rats per group is shown, and asterisks indicate that statistical analyses were performed using Fisher's exact test at 95% confidence. Values represent the mean ± SEM. (B and C) Depletion of CD25+ T reg cells from splenocytes derived from TBR restores tumor lysis and antigen–specific IFN-γ secretion. 104 PROb cells (or irrelevant glioma tumor cells) were cultured in the presence of 105 splenocytes depleted or not from CD25+ T reg cells and derived from TFR versus TBR at day 42. Cytotoxicity was determined with a crystal violet assay after 48 h of co-culture. (B) Increased tumor cell lysis on depletion of T reg cells. 105 T cells depleted or not from CD25+ T cells and derived from either TBR or naive rats were cultured in the presence of 104 CD11c+ DCs harvested from naive animals and pulsed with 104 PROb cell lysates. (C) Increased IFN-γ secretion on depletion of T reg cells. The accumulation of IFN-γ in the culture supernatant was determined by ELISA after 48 h of co-culture. Values represent the mean ± SEM (n = 3). Asterisks indicate significant differences (P < 0.05) using the Student's t test. This experiment has been repeated, yielding similar results.
Figure 4.
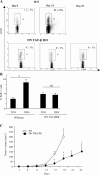
DCs from tumor bearers promote T reg cell proliferation in vitro. (A) Increased T reg cell proliferation induced by DCs from TBR. 105 CD25+ T cells and CD11b+ DCs were isolated from the spleen of either TFR or TBR and co-cultured alone or together at a 1:1 ratio for 5 d before pulsing with [3H]thymidine. (B) T reg cells maintain their inhibitory potential after stimulation by DCs from TBR. Spleen cells from a BD-IX rat were cultured for 5 d with mitomycin C–treated spleen cells from a Wistar rat, and BrdU was added during the last 12 h of the mixed lymphocyte culture before measuring BrdU incorporation. CD25+ T cells were isolated from tumor-bearing BD-IX rats and added at a ratio of 1:1, either immediately (+CD25+) or after a 3-d mixed culture with CD11b+ cells from TBR (+CD25+ expanded). (C) Requirement of class II and CD11b cells for T reg cell proliferation. 105 TCR+ cell-depleted splenocytes from TBR or TFR were further depleted from MHC class II+ cells or CD11b+ cells and co-cultured in the presence of 105 splenic CD25+ T cells from TBR (left) or TFR (right) for 5 d before pulsing with [3H]thymidine. Values represent the mean ± SEM of triplicate wells. The asterisks indicate statistical significance (P = 0.01) at 95% confidence using Fisher's exact test. (D) 105 CD11c− cells were isolated from the DLNs of TFM and TBM and co-cultured for 5 d with 105 spleen CD25− T cells from TBM before measuring [3H]thymidine incorporation. Values represent means ± SEM of triplicate wells. The asterisk indicates statistical significance (P = 0.005) at 95% confidence using Fisher's exact test.
Figure 5.
DCs from tumor bearers promote T reg cell proliferation in vivo. (A) Measurement of T reg cell proliferation elicited by DCs using BrdU. 2 × 106 CD11b+ DCs isolated from the spleen of TBR or TFR were injected into the left rear foot pad of TFR. 3 d later, BrdU was injected i.p. The percentages of BrdU+ T reg cells in the ipsi- versus contra-lateral LNs were determined by flow cytometry. The mean ± SEM of three rats per group are shown. The asterisk represent statistical significance (P = 0.02) at 95% confidence using the Mann-Whitney U test. (B) Measurement of T reg cell proliferation elicited by DCs using CFSE. 5 × 106 CFSE-labeled T reg cells isolated from the spleen of TBR were injected i.v. into TFR either alone (control) or in combination with 2 × 106 CD11b+ DC cells isolated from the spleen of TFR or TBR. 3 d later, CFSE dilution was studied in splenic CD4+ lymphocytes by flow cytometry. Percentages indicate the rate of proliferating cells. One representative out of two independent experiments is shown.
Figure 6.
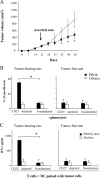
TGF-β signaling is critical for T reg cell proliferation and tumor escape. (A) Flow cytometry analyses of CD3+CD4+CD25+ cells isolated from the inguinal LNs of WT and DN TGF-βRII mice. The animals were either tumor free (0), or bore 15- or 25-d-old tumors. Data from one representative animal in each group is shown. Percentages of CD25 expression among CD3+CD4+ cells are shown. Values represent the mean ± SEM (n = 6). (B) BrdU incorporation into CD3+CD4+CD25+-gated T cells isolated from the inguinal LNs of WT and DN TGF-βRII mice. Animals were either tumor free or bore 15-d-old tumors, as indicated by the symbols, and the frequency of BrdU+ cells was determined. Values represent the mean ± SEM (n = 6). One representative experiment out of two is shown. The asterisk indicates statistical significance (P < 0.05) using the Student's t test. (C) Influence of the DN TGF-βRII transgene on PROb tumor growth. WT (open circles) and transgenic DN TGF-βRII mice (closed circles) were challenged s.c. with B16F10 cells, and tumor growth was monitored biweekly. One representative out of two experiments including six mice per group following the mean tumor volume ± SEM is depicted. The asterisk indicates statistical significance (P = 0.001) at 95% confidence using the Student's t test.
Figure 7.
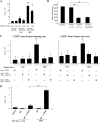
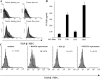
Tumor cells license IMDCs to produce TGF-β. (A) Intracellular staining of IMDCs from tumor-free or tumor-bearing rats and mice. CD11b+ DCs isolated from the spleen of TBR and TFR or CD11c+ IMDCs from DLNs of TFM or TBM were permeabilized and stained with a polyclonal anti–TGF-β antibody. Shaded histogram, anti–TGF-β; bold line histogram, isotype control. Note that no positive staining was obtained with nonpermeabilized cells (not depicted). (B) TGF-β secretion by IMDCs. IMDCs purified as in A were cultured for 48 h in serum-free medium and TGF-β1 levels in the supernatants were assessed by ELISA. Values represent the mean ± SEM (n = 3). (C) Induction of TGF-β production in mouse splenic IMDCs by tumor supernatants. CD11c+ DCs from inguinal LNs of TFM were cultured for 24 h in the absence or presence of B16F10 or NIH 3T3 culture supernatants or 10 ng/ml TGF-β1. Shaded histogram, test tube; open histogram, isotype control. At 24 h, TGF-β–expressing cells were identified by FACS analysis after permeabilization. The percentages of TGF-β expression cells are given. Data representative of two independent experiments are shown.
Figure 8.
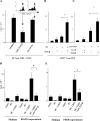
IMDCs stimulate T reg cell proliferation in a TGF-β–dependent fashion. (A) Neutralization of TGF-β curtails IMDC-driven T reg cell proliferation. 105 IMDCs were cultured for 5 d with T reg cells at a 1:1 ratio along with 1 ng/ml blocking anti–TGF-β antibody or an isotype-matched control before pulsing with [3H]thymidine. Both IMDCs and T reg cells were isolated from the spleen of TBR. The inset shows a similar experiment in which CD25+ splenic cells were labeled with CFSE, and CFSE expression in T cells was monitored by flow cytometry at day 5 (one representative of three experiments is shown). Values represent the mean ± SEM. The asterisk indicates statistical significance (P = 0.03). (B) Exogenous TGF-β cooperates with IMDCs from TFM to drive the proliferation of T reg cells. 105 splenic T reg cells from TBR were cultured in FBS-free medium supplemented with 200 UI/ml rIL-2 along with CD11b+ splenic DCs from tumor-free rats at a 1:1 ratio (left) in the presence of 1 ng/ml TGF-β1 before pulsing with [3H]thymidine. One representative out of five experiments is shown. Means and SEM of cpm of triplicate wells are shown. In all proliferation assays, CD11b+ DCs alone did not incorporate [3H]thymidine above background levels (not depicted). The asterisk indicates statistical significance (P < 0.05) using Fisher's exact test. (C) Stimulation of T reg cell proliferation by anti-CD3 and TGF-β1. T reg cells were stimulated with immobilized anti-CD3 and/or TGF-β1 (as in B). (D and E) In vitro cooperation between tumor cell supernatants and IMDCs to drive TGF-β–dependent T reg cell proliferation in mice (D) and in rats (E). 2 × 104 IMDCs isolated from tumor-free rodents and incubated in tumor cell supernatants for 24 h were co-cultured with 105 T reg cells from tumor-bearing rodents with anti–TGF-β1 blocking antibodies for 5 d. Proliferation of T reg cells was monitored in triplicate wells by measuring [3H]thymidine incorporation. One representative experiment out of three is depicted. The asterisk indicates statistical significance (P < 0.05) using Fisher's exact test. It is noteworthy that supernatants of NIH 3T3 did not license DCs for T reg cell proliferation (not depicted).
Similar articles
- TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets.
Pyzik M, Piccirillo CA. Pyzik M, et al. J Leukoc Biol. 2007 Aug;82(2):335-46. doi: 10.1189/jlb.1006644. Epub 2007 May 2. J Leukoc Biol. 2007. PMID: 17475784 - CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner.
Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. Ghiringhelli F, et al. J Exp Med. 2005 Oct 17;202(8):1075-85. doi: 10.1084/jem.20051511. J Exp Med. 2005. PMID: 16230475 Free PMC article. - Dendritic cells partially abrogate the regulatory activity of CD4+CD25+ T cells present in the human peripheral blood.
Ahn JS, Krishnadas DK, Agrawal B. Ahn JS, et al. Int Immunol. 2007 Mar;19(3):227-37. doi: 10.1093/intimm/dxl139. Epub 2007 Feb 7. Int Immunol. 2007. PMID: 17289657 - Type 1 T regulatory cells and their relationship with CD4+CD25+ T regulatory cells.
Roncarolo MG, Gregori S, Levings M. Roncarolo MG, et al. Novartis Found Symp. 2003;252:115-27; discussion 127-31, 203-10. Novartis Found Symp. 2003. PMID: 14609215 Review. - Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance.
Zhang L, Yi H, Xia XP, Zhao Y. Zhang L, et al. Autoimmunity. 2006 Jun;39(4):269-76. doi: 10.1080/08916930600753903. Autoimmunity. 2006. PMID: 16891215 Review.
Cited by
- Tumor-altered dendritic cell function: implications for anti-tumor immunity.
Hargadon KM. Hargadon KM. Front Immunol. 2013 Jul 11;4:192. doi: 10.3389/fimmu.2013.00192. eCollection 2013. Front Immunol. 2013. PMID: 23874338 Free PMC article. - Potential functional role of plasmacytoid dendritic cells in cancer immunity.
Kim R, Emi M, Tanabe K, Arihiro K. Kim R, et al. Immunology. 2007 Jun;121(2):149-57. doi: 10.1111/j.1365-2567.2007.02579.x. Epub 2007 Mar 20. Immunology. 2007. PMID: 17371541 Free PMC article. Review. - Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells.
Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Zhou Z, et al. Stem Cells. 2010 Mar 31;28(3):620-32. doi: 10.1002/stem.301. Stem Cells. 2010. PMID: 20073041 Free PMC article. - TGF-β signaling promotes tube-structure-forming growth in pancreatic duct adenocarcinoma.
Yamaguchi T, Ikehara S, Akimoto Y, Nakanishi H, Kume M, Yamamoto K, Ohara O, Ikehara Y. Yamaguchi T, et al. Sci Rep. 2019 Aug 2;9(1):11247. doi: 10.1038/s41598-019-47101-y. Sci Rep. 2019. PMID: 31375695 Free PMC article. - Pathological mobilization and activities of dendritic cells in tumor-bearing hosts: challenges and opportunities for immunotherapy of cancer.
Tesone AJ, Svoronos N, Allegrezza MJ, Conejo-Garcia JR. Tesone AJ, et al. Front Immunol. 2013 Dec 10;4:435. doi: 10.3389/fimmu.2013.00435. Front Immunol. 2013. PMID: 24339824 Free PMC article. Review.
References
- Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. - PubMed
- Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. - PubMed
- Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. - PubMed
- Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. - PubMed
- Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous