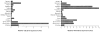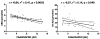Activation of the peripheral endocannabinoid system in human obesity - PubMed (original) (raw)
Activation of the peripheral endocannabinoid system in human obesity
Stefan Engeli et al. Diabetes. 2005 Oct.
Abstract
Obesity is the main risk factor for the development of type 2 diabetes. Activation of the central endocannabinoid system increases food intake and promotes weight gain. Blockade of the cannabinoid type 1 (CB-1) receptor reduces body weight in animals by central and peripheral actions; the role of the peripheral endocannabinoid system in human obesity is now being extensively investigated. We measured circulating endocannabinoid concentrations and studied the expression of CB-1 and the main degrading enzyme, fatty acid amide hydrolase (FAAH), in adipose tissue of lean (n = 20) and obese (n = 20) women and after a 5% weight loss in a second group of women (n = 17). Circulating levels of anandamide and 1/2-arachidonoylglycerol were increased by 35 and 52% in obese compared with lean women (P < 0.05). Adipose tissue mRNA levels were reduced by -34% for CB-1 and -59% for FAAH in obese subjects (P < 0.05). A strong negative correlation was found between FAAH expression in adipose tissue and circulating endocannabinoids. Circulating endocannabinoids and CB-1 or FAAH expression were not affected by 5% weight loss. The expression of CB-1 and FAAH was increased in mature human adipocytes compared with in preadipocytes and was found in several human tissues. Our findings support the presence of a peripheral endocannabinoid system that is upregulated in human obesity.
Figures
FIG. 1
Expression of CB-1 and FAAH genes in human tissues. Real-time RT-PCR of a multiple tissue human RNA panel, normalized by 18S rRNA expression. Data are given in arbitrary units (AUs) relative to CB-1 or FAAH gene expression in adipose tissue.
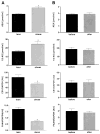
FIG. 2
Expression of CB-1 and FAAH by human adipose cells. A: CB-1 and FAAH mRNA was detected by real-time PCR in isolated human adipocytes. B: Confocal microscopy revealed localization of the CB-1 receptor in the adipocyte membrane. The negative control (left; only second antibody added) confirms the specificity of fluorescence signals seen in the right confocal image. C: Increased expression of CB-1 and FAAH genes in isolated mature human adipocytes (AC) compared with isolated human preadipocytes (PAC). CB-1 gene expression data were confirmed by Western blotting (insert). Data are means ± SE from seven pairs and are given as arbitrary units (AUs), normalized by 18S rRNA expression. Group comparison by Student’s t test. *P < 0.05.
FIG. 3
Influence of obesity and weight loss on the peripheral endocannabinoid system. Subcutaneous adipose tissue biopsies and blood samples were obtained from 20 lean and 20 obese postmenopausal women in a cross-sectional study (A) and from 17 obese postmenopausal women before and after a 5% body weight loss by a dietary protocol (B). Circulating levels of AEA and AGs (1/2-AG) were increased in the obese women by 35 and 52%, respectively. In contrast, adipose tissue CB-1 mRNA was decreased by −34% and FAAH mRNA by −59% in the obese subjects. Neither circulating levels of AEA and 1/2-AG nor adipose tissue, CB-1, or FAAH mRNA were influenced by the weight loss protocol. Gene expression is given in arbitrary units (AUs), normalized by GAPDH expression. Group comparison was performed with Student’s t test for independent samples (cross-sectional study) or the t test for paired samples (weight loss study). *P < 0.05 vs. lean.
FIG. 4
Relation of FAAH expression in adipose tissue and circulating endocannabinoids. Both circulating AEA and Ags (1/2-AG) were negatively correlated with the expression of the FAAH gene in 40 human adipose tissue samples. Gene expression is given in arbitrary units (AUs), normalized by GAPDH expression.
Similar articles
- Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity.
Blüher M, Engeli S, Klöting N, Berndt J, Fasshauer M, Bátkai S, Pacher P, Schön MR, Jordan J, Stumvoll M. Blüher M, et al. Diabetes. 2006 Nov;55(11):3053-60. doi: 10.2337/db06-0812. Diabetes. 2006. PMID: 17065342 Free PMC article. - Adipose tissue endocannabinoid system gene expression: depot differences and effects of diet and exercise.
You T, Disanzo BL, Wang X, Yang R, Gong D. You T, et al. Lipids Health Dis. 2011 Oct 28;10:194. doi: 10.1186/1476-511X-10-194. Lipids Health Dis. 2011. PMID: 22035053 Free PMC article. Clinical Trial. - Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects.
Engeli S, Lehmann AC, Kaminski J, Haas V, Janke J, Zoerner AA, Luft FC, Tsikas D, Jordan J. Engeli S, et al. Obesity (Silver Spring). 2014 May;22(5):E70-6. doi: 10.1002/oby.20728. Epub 2014 Mar 11. Obesity (Silver Spring). 2014. PMID: 24616451 Clinical Trial. - The endocannabinoid system: a new target for the regulation of energy balance and metabolism.
Després JP. Després JP. Crit Pathw Cardiol. 2007 Jun;6(2):46-50. doi: 10.1097/HPC.0b013e318057d4b4. Crit Pathw Cardiol. 2007. PMID: 17667864 Review.
Cited by
- AGMO Inhibitor Reduces 3T3-L1 Adipogenesis.
Fischer C, Wilken-Schmitz A, Hernandez-Olmos V, Proschak E, Stark H, Fleming I, Weigert A, Thurn M, Hofmann M, Werner ER, Geisslinger G, Niederberger E, Watschinger K, Tegeder I. Fischer C, et al. Cells. 2021 May 1;10(5):1081. doi: 10.3390/cells10051081. Cells. 2021. PMID: 34062826 Free PMC article. - Analysis of 14 endocannabinoids and endocannabinoid congeners in human plasma using column switching high-performance atmospheric pressure chemical ionization liquid chromatography-mass spectrometry.
Sempio C, Klawitter J, Jackson M, Freni F, Shillingburg R, Hutchison K, Bidwell LC, Christians U, Klawitter J. Sempio C, et al. Anal Bioanal Chem. 2021 May;413(12):3381-3392. doi: 10.1007/s00216-021-03280-0. Epub 2021 Apr 5. Anal Bioanal Chem. 2021. PMID: 33817753 - Peripheral effects of FAAH deficiency on fuel and energy homeostasis: role of dysregulated lysine acetylation.
Vaitheesvaran B, Yang L, Hartil K, Glaser S, Yazulla S, Bruce JE, Kurland IJ. Vaitheesvaran B, et al. PLoS One. 2012;7(3):e33717. doi: 10.1371/journal.pone.0033717. Epub 2012 Mar 19. PLoS One. 2012. PMID: 22442717 Free PMC article. - Gut Microbiota: Modulation of Host Physiology in Obesity.
Nehra V, Allen JM, Mailing LJ, Kashyap PC, Woods JA. Nehra V, et al. Physiology (Bethesda). 2016 Sep;31(5):327-35. doi: 10.1152/physiol.00005.2016. Physiology (Bethesda). 2016. PMID: 27511459 Free PMC article. Review. - Decreased circulating anandamide levels in preeclampsia.
Molvarec A, Fügedi G, Szabó E, Stenczer B, Walentin S, Rigó J Jr. Molvarec A, et al. Hypertens Res. 2015 Jun;38(6):413-8. doi: 10.1038/hr.2015.20. Epub 2015 Feb 26. Hypertens Res. 2015. PMID: 25716652
References
- Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. - PubMed
- Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–2079. - PubMed
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. - PubMed
- Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical