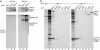Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane - PubMed (original) (raw)
Comparative Study
. 2005 Oct 4;102(40):14296-301.
doi: 10.1073/pnas.0505014102. Epub 2005 Sep 26.
Affiliations
- PMID: 16186509
- PMCID: PMC1242303
- DOI: 10.1073/pnas.0505014102
Comparative Study
Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane
Brendan N Lilley et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Polypeptides that fail to pass quality control in the endoplasmic reticulum (ER) are dislocated from the ER membrane to the cytosol where they are degraded by the proteasome. Derlin-1, a member of a family of proteins that bears homology to yeast Der1p, was identified as a factor that is required for the human cytomegalovirus US11-mediated dislocation of class I MHC heavy chains from the ER membrane to the cytosol. Derlin-1 acts in concert with the AAA ATPase p97 to remove dislocation substrate proteins from the ER membrane, but it is unknown whether other factors aid Derlin-1 in its function. Mammalian genomes encode two additional, related proteins (Derlin-2 and Derlin-3). The similarity of the mammalian Derlin-2 and Derlin-3 proteins to yeast Der1p suggested that these as-yet-uncharacterized Derlins also may play a role in ER protein degradation. We demonstrate here that Derlin-2 is an ER-resident protein that, similar to Derlin-1, participates in the degradation of proteins from the ER. Furthermore, we show that Derlin-2 forms a robust multiprotein complex with the p97 AAA ATPase as well as the mammalian orthologs of the yeast Hrd1p/Hrd3p ubiquitin-ligase complex. The data presented here define a set of interactions between proteins involved in dislocation of misfolded polypeptides from the ER.
Figures
Fig. 1.
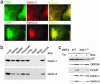
Derlins interact with VIMP. (a) Interactions of US11WT, class I HC, Derlin-1, and VIMP. Immunoprecipitations from digitonin lysates of US11WT or US11Q192L cell lines pulse-labeled for 30 min and chased for the indicated times were performed with anti-Derlin-1 (lanes 1-4) or anti-VIMP (lanes 5-8) Abs. The positions of the relevant polypeptides are indicated. (b) VIMP associates with Derlin proteins. U373 cells were labeled to steady state, and immunoprecipitations from digitonin lysates were performed with control rabbit IgG (lane 1) or anti-VIMP Abs (lanes 2-5). The anti-VIMP immunoprecipitate (IP) was either analyzed directly (lane 2) or subjected to reimmunoprecipitation (re-IP) in sequential fashion by using the Abs indicated.
Fig. 2.
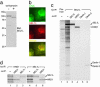
Characterization of Derlin-2. (a) Derlin-2 is an ER-resident protein. U373 cells were subjected to immunohistochemistry by using Abs against calnexin (CNX; green) and Derlin-1 or -2 (red) followed by confocal microscopy. The merged images are shown, and areas of colocalization are indicated in yellow. (b) Analysis of Derlin protein expression in mouse tissues by immunoblotting. Longer exposures of the immunoblot shown reveal low levels of Derlin-1 and -2 expression in heart and brain. (c) Induction of Derlin protein expression by the unfolded protein response. Shown is immunoblot analysis of WT or Xbp-1-deficient mouse embryonic fibroblasts (MEFs) either mock-treated or treated with 100 ng/ml tunicamycin (Tm) for 24 h. CHOP, CCAAT/enhancer-binding protein homologous protein.
Fig. 3.
Association of Derlin proteins with dislocation substrates and proteins involved in ER degradation. (a) U373 cells were pulse-labeled and then incubated without (-GST-Ub; lanes 1-3) or with (+GST-Ub; lanes 4-6) GST-ubiquitin. Immunoprecipitations (IPs) using the indicated Abs were either analyzed directly (Upper) or subjected to reimmunoprecipitation using anti-GST antibodies (Lower). The position of the stacking gel is indicated to demonstrate the presence of high-molecular-weight poly-GST-ubiquitinated material. (b) Immunoprecipitations from lysates of steady-state-labeled U373 cells were performed with control rabbit IgG (lane 1), anti-Derlin-1 Abs (lanes 2-8), or anti-Derlin-2 Abs (lanes 9-15). The respective immunoprecipitates were either analyzed directly (lanes 1, 2, and 9) or reimmunoprecipitated (re-IP) in sequential fashion using the indicated Abs.
Fig. 4.
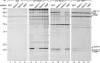
Knockdown of Derlin-1, Derlin-2, and VIMP reveals specificity of interactions. U373 cells expressing the indicated shRNA construct were labeled to steady state, and immunoprecipitations (IPs) were performed by using control rabbit IgG (rIgG; lanes 1-4), anti-Derlin-1 (lanes 5-8), anti-Derlin-2 (lanes 9-12), or anti-VIMP (lanes 13-16) Abs. The asterisks indicate nonspecifically bound polypeptides.
Fig. 5.
Analysis of SEL1L complexes. (a) Immunoblot of U373 cells either mock-treated or treated with 0.75 μg/ml tunicamycin for 14 h. (b) SEL1L largely colocalizes with calnexin (CNX) in U373 cells. (c) Immunoprecipitations (IPs) from lysates of steady-state-labeled U373 cells were performed with preimmune serum (lane 1) or anti-SEL1L Abs (lanes 2-6) and were either analyzed directly (lanes 1 and 2) or reimmunoprecipitated (re-IP) in sequential fashion using the indicated Abs. (d) As described for c except anti-HRD1 and anti-SEL1L Abs were used.
Fig. 6.
Map of interactions involved in the degradation of a subset of misfolded ER proteins. Previously demonstrated genetic or physical interactions observed in yeast compared with the physical interactions are shown here for the human orthologs. Interactions observed in high-throughput analyses or in analyses not pertaining to ER degradation are depicted separately from those observed in focused studies of ER protein degradation. The relative strengths of the interactions observed in this study are indicated by the thickness of the lines. The interaction maps are based on data either cited in the body of the text or from the Incyte Genomics Proteome Bioknowledge Library (39, 50) and references therein. The Der1p pathway only processes a subset of substrate proteins in yeast. Currently, the extent to which the Derlin-1 and Derlin-2 pathways are used in mammalian cells is not known.
Similar articles
- A membrane protein required for dislocation of misfolded proteins from the ER.
Lilley BN, Ploegh HL. Lilley BN, et al. Nature. 2004 Jun 24;429(6994):834-40. doi: 10.1038/nature02592. Nature. 2004. PMID: 15215855 - Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane.
Ye Y, Shibata Y, Kikkert M, van Voorden S, Wiertz E, Rapoport TA. Ye Y, et al. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14132-8. doi: 10.1073/pnas.0505006102. Epub 2005 Sep 26. Proc Natl Acad Sci U S A. 2005. PMID: 16186510 Free PMC article. - Derlin-1 and p97/valosin-containing protein mediate the endoplasmic reticulum-associated degradation of human V2 vasopressin receptors.
Schwieger I, Lautz K, Krause E, Rosenthal W, Wiesner B, Hermosilla R. Schwieger I, et al. Mol Pharmacol. 2008 Mar;73(3):697-708. doi: 10.1124/mol.107.040931. Epub 2007 Nov 29. Mol Pharmacol. 2008. PMID: 18048502 - Mechanism and components of endoplasmic reticulum-associated degradation.
Hoseki J, Ushioda R, Nagata K. Hoseki J, et al. J Biochem. 2010 Jan;147(1):19-25. doi: 10.1093/jb/mvp194. Epub 2009 Nov 18. J Biochem. 2010. PMID: 19923195 Review. - p97/p47-Mediated biogenesis of Golgi and ER.
Uchiyama K, Kondo H. Uchiyama K, et al. J Biochem. 2005 Feb;137(2):115-9. doi: 10.1093/jb/mvi028. J Biochem. 2005. PMID: 15749824 Review.
Cited by
- The intrinsically disordered membrane protein selenoprotein S is a reductase in vitro.
Liu J, Li F, Rozovsky S. Liu J, et al. Biochemistry. 2013 May 7;52(18):3051-61. doi: 10.1021/bi4001358. Epub 2013 Apr 24. Biochemistry. 2013. PMID: 23566202 Free PMC article. - Identification of two rate-limiting steps in the degradation of partially folded immunoglobulin light chains.
Mann MJ, Flory AR, Oikonomou C, Hayes CA, Melendez-Suchi C, Hendershot LM. Mann MJ, et al. Front Cell Dev Biol. 2022 Aug 22;10:924848. doi: 10.3389/fcell.2022.924848. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36072336 Free PMC article. - A slowly cleaved viral signal peptide acts as a protein-integral immune evasion domain.
Seidel E, Dassa L, Kahlon S, Tirosh B, Halenius A, Seidel Malkinson T, Mandelboim O. Seidel E, et al. Nat Commun. 2021 Apr 6;12(1):2061. doi: 10.1038/s41467-021-21983-x. Nat Commun. 2021. PMID: 33824318 Free PMC article. - The human selenoprotein VCP-interacting membrane protein (VIMP) is non-globular and harbors a reductase function in an intrinsically disordered region.
Christensen LC, Jensen NW, Vala A, Kamarauskaite J, Johansson L, Winther JR, Hofmann K, Teilum K, Ellgaard L. Christensen LC, et al. J Biol Chem. 2012 Jul 27;287(31):26388-99. doi: 10.1074/jbc.M112.346775. Epub 2012 Jun 14. J Biol Chem. 2012. PMID: 22700979 Free PMC article. - The enigmatic ATP supply of the endoplasmic reticulum.
Depaoli MR, Hay JC, Graier WF, Malli R. Depaoli MR, et al. Biol Rev Camb Philos Soc. 2019 Apr;94(2):610-628. doi: 10.1111/brv.12469. Epub 2018 Oct 19. Biol Rev Camb Philos Soc. 2019. PMID: 30338910 Free PMC article. Review.
References
- Hirsch, C., Jarosch, E., Sommer, T. & Wolf, D. H. (2004) Biochim. Biophys. Acta 1695, 215-223. - PubMed
- Gavin, A. C., Bosche, M., Krause, R., Grandi, P., Marzioch, M., Bauer, A., Schultz, J., Rick, J. M., Michon, A. M., Cruciat, C. M., et al. (2002) Nature 415, 141-147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials