Multiple myeloma in a murine syngeneic model:modulation of growth and angiogenesis by a monoclonal antibody to kininogen - PubMed (original) (raw)
Multiple myeloma in a murine syngeneic model:modulation of growth and angiogenesis by a monoclonal antibody to kininogen
Irma M Sainz et al. Cancer Immunol Immunother. 2006 Jul.
Abstract
Multiple myeloma (MM), a B-cell malignancy characterized by proliferation of monoclonal plasma cells remains incurable. Murine plasma cell tumors share common features with human MM. We used two cell lines (B38 and C11C1) derived from P3X63Ag8 myeloma cells. The new cell lines were implanted subcutaneously in the strain of origin (Balb/c mice) and used as a model to monitor the effects of C11C1 monoclonal antibody (mAb) to kininogen (HK). We assessed their behavior by intraperitoneal and subcutaneous implantation, by implanting them together and by treating B38-MM with purified mAb C11C1. We evaluated growth, microvascular density (MVD), and cellular expression of urokinase-type plasminogen activator-receptor (uPAR), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), bradykinin-1 receptor (B1R), bradykinin-2 receptor (B2R) and HK. We found that both MM-cell-lines are uPAR positive, that mAb C11C1 inhibits its own tumor growth in vivo, slows down B38-MM growth rate when both MM are implanted together and when mAb C11C1 is injected intraperitoneally. MAb C11C1-treated-MM showed decreased MVD and HK binding in vivo without FGF-2, B1R or B2R expression changes. We propose that the B38-extramedullary-myeloma-model is a useful tool to study the interactions of this hematopoietic tumor and its environment and that mAb C11C1 may improve the efficacy of conventional MM treatment with minimal side effects.
Figures
Fig. 1
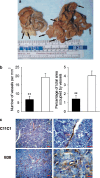
Murine MM, intraperitoneal growth and microvascular density. a. IP growth. Representative gross abdominal findings at necropsy from specimens collected on maximum growth (B38-MM: day 10 and C11C1-MM: day 28). The black arrows point to the myelomatous growth in both specimens. C11C1 tumor nodules were clearly decreased in size and number. b Microvascular density (MVD) C11C1-MM solid bars; B38-MM open bars. The mean ± SEM number of vessels per mm2 (left) and percentage of area occupied by vessels (right) in each group is shown. MVD was significantly decreased in sections from the C11C1-MM group (_n_=3) when compared to B38-MM group (_n_=5).**, _P_= 0.01. c Immunohistochemistry of endothelial cells. Representative microphotographs (left: x400, right: x1000) from tumor sections showing blood vessel walls (arrows) stained golden-brown to black. The sections were incubated with an antibody to human vWF that crossreacts with the mouse molecule, and counterstained with hematoxylin. _Red bar_= 50 μm. C11C1-MM group has significantly fewer vessels than the B38-MM group
Fig. 2
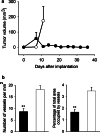
Murine MM, subcutaneous growth and MVD. a Growth: Values shown are mean ± SEM. Group B38 (open circles) (_n_=10) reached the 2 cm length limit by day 11. C11C1 group (solid circles) (_n_=20) did not reach the length limit. The C11C1 tumors not excised regressed by day 22. Statistical comparisons made with day 11 data (_P_=0.02). b MVD C11C1 solid bars; B38 open bars. The mean ± SEM of the number of vessels per mm2 (left) and the percentage of total area occupied by vessels (right) were statistically significant. **_P_=0.01.
Fig. 3
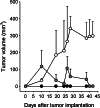
Murine MM, combined subcutaneous growth: Combined group C11C1 and B38 (gray hexagons) received C11C1-MM and B38-MM cells which were implanted together in the back of Balb/c mice (n=5) to monitor their growth. Group B38 (open circles) (_n_=5) and group C11C1 (solid circles) (_n_=5) received either B38-MM or C11C1-MM cells (implanted in the back of Balb/c mice) and were used to monitor their separate growth rate in vivo. There were no significant differences up to and included day 26 between the combined group and the B38 group. At day 28 and beyond the B38 group showed a statistically significant increase in volume when compared to the combined group (P<0.05). The B38 group tumor volume was significantly larger than the C11C1 group from day 10 and beyond (P<0.05). Values shown are mean ± SEM.
Fig. 4
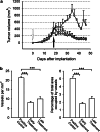
Murine MM, subcutaneous growth and early and late treatment. a Growth: All animals were implanted with B38-MM cells. The untreated control group (circles) showed a steady growth rate until day 32. Animals that received purified mAb C11C1 for early treatment from day zero (open arrow; diamonds) showed a statistically significant decrease in tumor volume from the untreated control group starting from day 28 (P<0.05). Animals from the late-treatment group started mAb C11C1 intraperitoneal injections on day 18 (solid arrow; squares) showed statistically significant decrease in tumor volume on day 30 and afterwards (P<0.05). Values shown are mean ± SEM. b B38-MM early- and late-treatment MVD: The mean ± SEM of the number of vessels per mm2 (left) and the percentage of total area occupied by vessels (right) were statistically significant between the control and both treatment groups (P<0.005). There was no statistically significant difference between the treatment groups (_P_=0.01). ***P<0.005
Fig. 5
VEGF and HK immunohistochemistry: magnification: left ×400, right ×1000; red and _green bars_= 50 micrometers; positive staining is indicated by brown pigment within cytoplasm. VEGF (left panel): red arrows point to malignant tumor cells; yellow arrows point to stromal tissue (connective tissue, endothelial cells); The positive control group showed 5.2±0.9% of tumor and stromal cells positive to VEGF while both treatment groups showed more than 90% immunopositivity. HK (right panel): orange arrows point to HK-positive endothelial cells; green arrows point to HK-negative endothelial cells. The positive control group showed more than 75% of endothelial cell immunopositivity while both treatment groups showed significantly less HK-positive endothelial cells (early treatment: 27.2±1.8%; late treatment: 42.3±7.4%). There was no difference in immunopositivity for uPAR, FGF-2, BIR, and B2R among all experimental groups
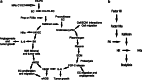
Fig. 6
The Plasma Kallikrein–Kinin System and mAb C11C1 effect in the angiogenic cascade. a C11C1 mAb and the angiogenic cascade. MAb C11C1 C11C1 monoclonal antibody. HK high-molecular-weight kininogen. PK prekallikrein. EC endothelial cell. Prcp prolylcarboxypeptidase. FXIIa activated coagulation factor XII. HKa cleaved high-molecular-weight kininogen. IL-8 interleukin 8. BK bradykinin. B2R bradykinin 2 receptor. eNOS endothelial cell nitric oxide system. VEGF: vascular endothelial growth factor. IL-6 interleukin 6. FGF-2 fibroblast growth factor 2, basic fibroblast growth factor. ECM extracellular matrix. uPA urokinase type plasminogen activator. The plasma complex HK–PK binds to the endothelial cells, where PK is activated to kallikrein (by either Prcp or FXIIa). Kallikrein cleaves HK releasing HKa and BK. BK stimulates IL-8 secretion (a potent angiogenic and tumor growth inducer) and the production and secretion of eNOS through the B2R on the surface of stromal and tumor cells. eNOS induces endothelial cell release of VEGF and endothelial cell proliferation and migration. VEGF induces eNOS and IL-6 release (which promotes tumor growth). Kallikrein also cleaves prourokinase to urokinase initiating a different pathway. Urokinase is involved in cell/ECM interactions favoring tumor spread and endothelial cell migration. Urokinase also activates plasminogen into plasmin and the proteolysis of the extracellular matrix releasing FGF-2. FGF-2 induces endothelial cell migration and the release of IL-6, which promotes tumor growth. MAb C11C1 inhibits the adhesion of HK and/ or the HK–PK complex to the endothelial cells, inhibiting the BK and uPA activation through kallikrein thus inhibiting tumor angiogenesis and growth. b Plasma Kallikrein-Kinin system: Consists of three proteins, Factor XII (FXII), Prekallikrein (PK), and high-molecular-weight kininogen (HK). Activated FXII (FXIIa) cleaves PK to kallikrein; kallikrein cleaves HK releasing BK and activated HK (HKa)
Similar articles
- Inhibition of metastasis of syngeneic murine melanoma in vivo and vasculogenesis in vitro by monoclonal antibody C11C1 targeted to domain 5 of high molecular weight kininogen.
Khan ST, Pixley RA, Liu Y, Bakdash N, Gordon B, Agelan A, Huang Y, Achary MP, Colman RW. Khan ST, et al. Cancer Immunol Immunother. 2010 Dec;59(12):1885-93. doi: 10.1007/s00262-010-0915-0. Epub 2010 Sep 2. Cancer Immunol Immunother. 2010. PMID: 20811885 Free PMC article. - The contact system and angiogenesis: potential for therapeutic control of malignancy.
Colman RW. Colman RW. Semin Thromb Hemost. 2004 Feb;30(1):45-61. doi: 10.1055/s-2004-822970. Semin Thromb Hemost. 2004. PMID: 15034797 Review. - Inhibition of tumor angiogenesis in vivo by a monoclonal antibody targeted to domain 5 of high molecular weight kininogen.
Song JS, Sainz IM, Cosenza SC, Isordia-Salas I, Bior A, Bradford HN, Guo YL, Pixley RA, Reddy EP, Colman RW. Song JS, et al. Blood. 2004 Oct 1;104(7):2065-72. doi: 10.1182/blood-2004-02-0449. Epub 2004 May 25. Blood. 2004. PMID: 15161672 - Inhibition of angiogenesis by antibody blocking the action of proangiogenic high-molecular-weight kininogen.
Colman RW, Pixley RA, Sainz IM, Song JS, Isordia-Salas I, Muhamed SN, Powell JA Jr, Mousa SA. Colman RW, et al. J Thromb Haemost. 2003 Jan;1(1):164-70. doi: 10.1046/j.1538-7836.2003.00025.x. J Thromb Haemost. 2003. PMID: 12871554 - Regulation of angiogenesis by the kallikrein-kinin system.
Colman RW. Colman RW. Curr Pharm Des. 2006;12(21):2599-607. doi: 10.2174/138161206777698710. Curr Pharm Des. 2006. PMID: 16842160 Review.
Cited by
- Antibody-based therapies in multiple myeloma.
Tai YT, Anderson KC. Tai YT, et al. Bone Marrow Res. 2011;2011:924058. doi: 10.1155/2011/924058. Epub 2011 Mar 2. Bone Marrow Res. 2011. PMID: 22046572 Free PMC article. - Inhibition of metastasis of syngeneic murine melanoma in vivo and vasculogenesis in vitro by monoclonal antibody C11C1 targeted to domain 5 of high molecular weight kininogen.
Khan ST, Pixley RA, Liu Y, Bakdash N, Gordon B, Agelan A, Huang Y, Achary MP, Colman RW. Khan ST, et al. Cancer Immunol Immunother. 2010 Dec;59(12):1885-93. doi: 10.1007/s00262-010-0915-0. Epub 2010 Sep 2. Cancer Immunol Immunother. 2010. PMID: 20811885 Free PMC article. - Polyclonal rabbit anti-murine plasmacytoma cell globulins induce myeloma cells apoptosis and inhibit tumour growth in mice.
Mu B, Yang JL, Gou LT, Yao YQ, Zhou Y, Cheng ZH, Shi HS, Li ZY, Wen Y, Leng F, Cui FY, Ma TT, Wei YQ. Mu B, et al. Apoptosis. 2011 Apr;16(4):370-81. doi: 10.1007/s10495-010-0568-7. Apoptosis. 2011. PMID: 21197579 Free PMC article. - u-PAR expression in cancer associated fibroblast: new acquisitions in multiple myeloma progression.
Ciavarella S, Laurenzana A, De Summa S, Pilato B, Chillà A, Lacalamita R, Minoia C, Margheri F, Iacobazzi A, Rana A, Merchionne F, Fibbi G, Del Rosso M, Guarini A, Tommasi S, Serratì S. Ciavarella S, et al. BMC Cancer. 2017 Mar 24;17(1):215. doi: 10.1186/s12885-017-3183-y. BMC Cancer. 2017. PMID: 28340565 Free PMC article.
References
- Criteria for the classification of monoclonal gammopathies multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57. - PubMed
- Roschke V, Hausner P, Kopantzev E, Pumphrey JG, Riminucci M, Hilbert DM, Rudikoff S. Disseminated growth of murine plasmacytoma: similarities to multiple myeloma. Cancer Res. 1998;58:535–541. - PubMed
- Song JS, Sainz IM, Cosenza SC, Isordia-Salas I, Bior A, Bradford HN, Guo YL, Pixley RA, Reddy EP, Colman RW. Inhibition of tumor angiogenesis in vivo by a monoclonal antibody targeted to domain 5 of high molecular weight kininogen. Blood. 2004;104:2065–2072. doi: 10.1182/blood-2004-02-0449. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA083121/CA/NCI NIH HHS/United States
- T32 HL007777/HL/NHLBI NIH HHS/United States
- T32 HL-07777-14/HL/NHLBI NIH HHS/United States
- R01 CA-083121-08/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical