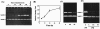Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis - PubMed (original) (raw)
Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis
Meng-Hsing Wu et al. Am J Pathol. 2005 Oct.
Abstract
Decreased phagocytotic ability of macrophages has been reported to be associated with the severity of endometriosis, although the underlying mechanism remains uncharacterized. Expression and secretion of matrix metalloproteinase (MMP)-9 by macrophages is a means to degrade the extracellular matrix of cells that are designated for phagocytosis. Here, we describe the regulation of MMP-9 expression and activity in peritoneal macrophages of women with endometriosis. Results demonstrated that peritoneal macrophages isolated from women with endometriosis have decreased levels of protein and enzyme activity of MMP-9. Treatment of macrophages with peritoneal fluid obtained from patients with severe endometriosis inhibited MMP-9 expression and gelatinase activity. Further investigation identified prostaglandin (PG) E(2) as the major factor in the peritoneal fluid that inhibited MMP-9 activity. The inhibitory effect of PGE(2) was mediated via the EP2/EP4-dependent PKA pathway. Furthermore, expression of tissue inhibitor of metalloproteinase-1, tissue inhibitor of metalloproteinase-2, and RECK in macrophages was not affected by treatment with PGE(2), indicating the effect of PGE(2) on suppressing MMP-9 activity was not mediated by up-regulation of its inhibitor. Our results suggest that decreased phagocytotic capability of peritoneal macrophage in patients with endometriosis may be caused by PGE(2)-mediated decreases in MMP-9 expression.
Figures
Figure 1
Distinct MMP-9 expression patterns in peritoneal macrophages of women with or without endometriosis. A: A representative gel picture showing RT-PCR result of levels of MMP-9 mRNA in macrophages isolated from PF of normal (N), early-stage endometriosis (EE), and severe-stage endometriosis (SE) women. GAPDH was amplified to serve as an internal control. NC, negative control omitting reverse transcriptase. B: A representative Western blot result demonstrates that expression of MMP-9 protein is decreased along with the severity of endometriosis. The band intensities of MMP-9 and β-actin were quantified using AlphaImager software and analyzed. C: The means and SE of ratios of MMP-9 to β-actin obtained from 7 normal patients (N), 8 early-stage endometriosis (EE), and 10 severe-stage endometriosis (SE) patients. Different letters denote significant differences between groups when Tukey’s analysis yields P < 0.05.
Figure 2
Gelatinase activity of MMP-9 but not MMP-2 was decreased in peritoneal macrophages isolated from patients with endometriosis. A and B: Analysis of the time effect on the activation of peritoneal macrophages in terms of gelatinase activity. Macrophages (2 × 105 cells/well) isolated from patients with endometriosis were plated in 24-well plates for the indicated time and culture media were collected for zymographic analysis. A: A representative zymographic picture revealed that the enzymatic activity of MMP-9 secreted by peritoneal macrophages was elevated along with time of culture. B: The means of relative intensity units of MMP-9 obtained from four different samples. Because the gelatinase activity proportionally increases between 12 and 24 hours after attachment, we thus empirically chose 16 hours as the time for all of the following experiments. C: A representative zymographic picture shows a decrease in MMP-9 but not MMP-2 enzymatic activity along with the severity of endometriosis. Six batches of peritoneal macrophages isolated from normal (N), early-stage endometriosis (EE), and severe-stage endometriosis (SE) women, respectively, were subjected to zymographic analyses and the results were similar. D: A representative zymographic picture shows gelatinase activity of MMP-9 in cultured media of macrophages isolated from PF and peripheral blood (PB). Three batches of macrophages isolated from peripheral blood of normal (N), early-stage endometriosis (EE), and severe-stage endometriosis (SE) women were subjected to zymographic analyses and the results were similar.
Figure 3
Endometriotic PFs suppressed gelatinase activities of MMP-9 in peritoneal macrophages. A and C: Gelatinase activity of normal and endometriosis peritoneal macrophages treated by normal peritoneal fluids, PF(N), and endometriotic peritoneal fluids, PF(E). Macrophages (2 × 105 cells/well) isolated from patients were plated in 24-well plates and treated by PF(N) and PF(E) with 1:1, 1:5, and 1:10 dilution. Representative zymographic pictures show that enzymatic activity of MMP-9 secreted by normal (A) or endometriotic (C) peritoneal macrophages treated with PF obtained normal (N) and/or endometriosis (Endo) patients. B and D: Means and SE of relative gelatinase activities of MMP-9 obtained from five and six experiments using different batches of cells obtained from normal and endometriosis patients, respectively. Asterisks denote significant difference from the control group.
Figure 4
The effect of PFs on expression of MMP-9 and MMP inhibitors in peritoneal macrophages. Macrophages were plated for 16 hours and then treated with peritoneal fluids from normal, PF(N), or endometriotic, PF(E), patients for 24 hours as described in the Materials and Methods. A: A representative gel picture showing RT-PCR result of levels of TIMP-1, TIMP-2, and RECK mRNA in macrophages. This experiment was repeated four times and the results were similar. B: A representative Western blot result demonstrates the expression of TIMP-1, TIMP-2, and β-actin protein. This experiment was repeated three times and the results were similar. C: A representative gel picture showing RT-PCR result of levels of MMP-9 mRNA in endometriotic PF-treated macrophages. Expression of MMP-9 mRNA was decreased by treatment with endometriotic PF in normal (N, n = 3), early-stage endometriosis (EE, n = 3), and severe-stage endometriosis (SE, n = 6) patients, respectively. D: A representative Western blot shows a significant decrease in expression of MMP-9 protein in peritoneal macrophages obtained from normal women treated with endometriotic PF (top). Endometriotic PF (without macrophages) was used as a negative control to demonstrate the presence of MMP-9 that was produced by macrophage but not from PF. The bottom panel shows means and standard errors of four experiments using different batches of cells. Asterisks denote results significantly different from the control group.
Figure 5
Influence of gelatinase activity of MMP-9 by cytokines and proinflammatory agents. A: Zymographic pictures showing gelatinase activity of MMP-9 secreted by peritoneal macrophages. MMP-9 activities were slightly increased by IL-1β treatment, whereas IFN-γ treatment exhibited inhibition at high dose (200 ng/ml). Treatment with leptin revealed no significant difference in MMP-9 activity. All of the experiments were repeated at least four times and the results were similar. B: A representative zymographic picture showing PGE2 treatment significantly inhibited MMP-9 activity in a dose-dependent manner. The experiment was repeated four times and the results were similar.
Figure 6
Effect of PGE2 on expression of MMP-9 and MMP inhibitors in peritoneal macrophages. Peritoneal macrophages were treated with 1 or 10 μmol/L PGE2. A: A representative gel picture showing RT-PCR result of levels of TIMP-1, TIMP-2, and RECK mRNA in macrophages. The experiment was repeated four times and the results were similar. B: A representative Western blot result demonstrates the expression of TIMP-1, TIMP-2, and β-actin protein. The experiment was repeated three times and the results were similar. C: A representative gel picture showing RT-PCR result of levels of MMP-9 mRNA in PGE2-treated macrophages. Expression of MMP-9 mRNA was decreased by treatment with 1 and 10 μmol/L PGE2, respectively. The experiment was repeated four times and the results were similar. D: Western blot analyses revealed a significant decrease in expression of MMP-9 protein with PGE2 treatment in peritoneal macrophages. The experiment was repeated four times and the results were similar.
Figure 7
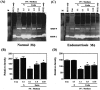
Effect of PGE2 receptor agonist and/or antagonist treatment on gelatinase activity and expression of MMP-9 in peritoneal macrophages. A: A representative Western blot shows expression of MMP-9 protein in macrophages treated with PGE2 receptor agonist and/or antagonist. Macrophages were treated with EP2 agonist (butaprost, buta), EP3 receptor agonist (sulprostone, Sul), or a combination of EP2/EP4 receptor agonist, PGE1OH, and EP1/EP2 receptor antagonist, AH6809 (E1OH+AH), and expression of MMP-9 protein was analyzed. The bottom panel shows means and standard errors obtained from four experiments in which different batches of cells were used. Asterisks denote significant difference from control group (P < 0.05). B: A representative gel picture shows RT-PCR results of levels of EP2, EP3, EP4, and GAPDH mRNA in peritoneal macrophages (labeled as 1 to 4). C: A representative Western blot result demonstrating the expression of MMP-9 protein in macrophages treated with different EP receptor agonists and/or antagonist. This experiment was repeated three times and the results were similar. D: Zymographic picture shows gelatinase activity of MMP-9 secreted by peritoneal macrophages. Pretreatment with H89 reversed PGE2- and butaprost-suppressed MMP-9 gelatinase activity. This experiment was repeated three times and the results were similar.
Figure 8
The schematic drawing indicates expression of MMP-9 by macrophages was suppressed by factors such as PGE2 present in the PF of women with endometriosis.
Similar articles
- Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: evidence for the role of TNF-alpha and cyclooxygenase-2.
Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Steenport M, et al. J Immunol. 2009 Dec 15;183(12):8119-27. doi: 10.4049/jimmunol.0901925. J Immunol. 2009. PMID: 19923455 Free PMC article. - Suppression of annexin A2 by prostaglandin E₂ impairs phagocytic ability of peritoneal macrophages in women with endometriosis.
Wu MH, Chuang PC, Lin YJ, Tsai SJ. Wu MH, et al. Hum Reprod. 2013 Apr;28(4):1045-53. doi: 10.1093/humrep/det003. Epub 2013 Jan 22. Hum Reprod. 2013. PMID: 23340055 - Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis.
Chuang PC, Lin YJ, Wu MH, Wing LY, Shoji Y, Tsai SJ. Chuang PC, et al. Am J Pathol. 2010 Feb;176(2):850-60. doi: 10.2353/ajpath.2010.090551. Epub 2009 Dec 24. Am J Pathol. 2010. PMID: 20035060 Free PMC article. - 15-Epi-lipoxin A(4) inhibits the progression of endometriosis in a murine model.
Chen QH, Zhou WD, Pu DM, Huang QS, Li T, Chen QX. Chen QH, et al. Fertil Steril. 2010 Mar 15;93(5):1440-7. doi: 10.1016/j.fertnstert.2009.01.107. Epub 2009 Mar 6. Fertil Steril. 2010. PMID: 19268934
Cited by
- The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target.
Ke J, Ye J, Li M, Zhu Z. Ke J, et al. Biomolecules. 2021 Nov 22;11(11):1739. doi: 10.3390/biom11111739. Biomolecules. 2021. PMID: 34827737 Free PMC article. Review. - Immunological aspects of endometriosis: a review.
Králíčková M, Vetvicka V. Králíčková M, et al. Ann Transl Med. 2015 Jul;3(11):153. doi: 10.3978/j.issn.2305-5839.2015.06.08. Ann Transl Med. 2015. PMID: 26244140 Free PMC article. Review. - Endometriosis-Associated Macrophages: Origin, Phenotype, and Function.
Hogg C, Horne AW, Greaves E. Hogg C, et al. Front Endocrinol (Lausanne). 2020 Jan 23;11:7. doi: 10.3389/fendo.2020.00007. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32038499 Free PMC article. Review. - Co-micronized Palmitoylethanolamide/Polydatin Treatment Causes Endometriotic Lesion Regression in a Rodent Model of Surgically Induced Endometriosis.
Di Paola R, Fusco R, Gugliandolo E, Crupi R, Evangelista M, Granese R, Cuzzocrea S. Di Paola R, et al. Front Pharmacol. 2016 Oct 14;7:382. doi: 10.3389/fphar.2016.00382. eCollection 2016. Front Pharmacol. 2016. PMID: 27790149 Free PMC article. - The Role of Peritoneal Macrophages in Endometriosis.
Ramírez-Pavez TN, Martínez-Esparza M, Ruiz-Alcaraz AJ, Marín-Sánchez P, Machado-Linde F, García-Peñarrubia P. Ramírez-Pavez TN, et al. Int J Mol Sci. 2021 Oct 6;22(19):10792. doi: 10.3390/ijms221910792. Int J Mol Sci. 2021. PMID: 34639133 Free PMC article. Review.
References
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–425. - PubMed
- Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S. Estrogen production and metabolism in endometriosis. Ann NY Acad Sci. 2002;955:75–85. - PubMed
- Tsai SJ, Wu MH, Lin CC, Sun HS, Chen SM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86:5765–5773. - PubMed
- Dmowski WP, Braun D, Gebel H. Endometriosis: genetic and immunologic aspects. Prog Clin Biol Res. 1990;323:99–122. - PubMed
- Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous