A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle - PubMed (original) (raw)
Comparative Study
A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle
Lydie Combaret et al. J Physiol. 2005.
Abstract
We tested the hypothesis that skeletal muscle ubiquitin-proteasome-dependent proteolysis is dysregulated in ageing in response to feeding. In Experiment 1 we measured rates of proteasome-dependent proteolysis in incubated muscles from 8- and 22-month-old rats, proteasome activities, and rates of ubiquitination, in the postprandial and postabsorptive states. Peptidase activities of the proteasome decreased in the postabsorptive state in 22-month-old rats compared with 8-month-old animals, while the rate of ubiquitination was not altered. Furthermore, the down-regulation of in vitro proteasome-dependent proteolysis that prevailed in the postprandial state in 8-month-old rats was defective in 22-month-old rats. Next, we tested the hypothesis that the ingestion of a 5% leucine-supplemented diet may correct this defect. Leucine supplementation restored the postprandial inhibition of in vitro proteasome-dependent proteolysis in 22-month-old animals, by down-regulating both rates of ubiquitination and proteasome activities. In Experiment 2, we verified that dietary leucine supplementation had long-lasting effects by comparing 8- and 22-month-old rats that were fed either a leucine-supplemented diet or an alanine-supplemented diet for 10 days. The inhibited in vitro proteolysis was maintained in the postprandial state in the 22-month-old rats fed the leucine-supplemented diet. Moreover, elevated mRNA levels for ubiquitin, 14-kDa ubiquitin-conjugating enzyme E2, and C2 and X subunits of the 20S proteasome that were characteristic of aged muscle were totally suppressed in 22-month-old animals chronically fed the leucine-supplemented diet, demonstrating an in vivo effect. Thus the defective postprandial down-regulation of in vitro proteasome-dependent proteolysis in 22-month-old rats was restored in animals chronically fed a leucine-supplemented diet.
Figures
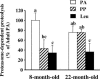
Figure 1. Skeletal muscle proteasome-dependent proteolysis after feeding 8- and 22-month-old animals
Rats from Experiment 1 were overnight starved (PA), or fed during 1 h either a alanine-supplemented (PP) or a leucine-supplemented meal (Leu). Proteasome-dependent proteolysis was measured as indicated in Methods in incubated epitrochlearis muscles harvested in the PA state or 2 h after meal ingestion. Values are means ±
s.e.m.
(vertical bars) for 9–10 animals and are expressed as a percentage of 8-month-old PA. Columns with different letters are significantly different from each other as assessed by ANOVA (P < 0.05).
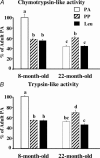
Figure 2. Chymotrypsin-like (A), and trypsin-like (B) peptidase activities of the proteasome in pooled extensor digitorum longus muscles (n = 7–10 rats) from 8- and 22-month-old rats
Animals from Experiment 1 were overnight starved (PA) or fed during 1 h (PP or Leu) as described in Fig. 1 legend. Data represent the slopes of best fit of arbitrary fluorescence units released from Suc-LLVY-AMC (chymotrypsin-like activity) or Boc-LRR-AMC (trypsin-like activity) versus time. Data are expressed as percentage of 8-month-old PA and bars denote standard errors of the slopes. Columns with different letters are significantly different by comparing the slopes of best fit (P < 0.05).
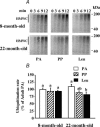
Figure 3. Ubiquitination rates in pooled extensor digitorum longus muscles (n = 9–11 rats) from 8- and 22-month-old rats
The formation of high molecular weight [125I]Ub conjugates (HMWC) in extensor digitorum longus muscle extracts was followed by autoradiography (A). Animals from Experiment 1 were overnight starved (PA) or fed during 1 h (PP or Leu) as described in Fig. 1 legend. B, the comparison of ubiquitination rates (e.g. the slopes of best fit of cpm bound to HMWC following gel excision versus time). Data are expressed as percentage of 8-month-old PA and bars denote standard errors of the slopes. Columns with different letters are significantly different by comparing the slopes of best fit (P < 0.05).
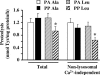
Figure 4. Total and non-lysosomal Ca2+-independent proteolysis in 22-month-old rats chronically fed an alanine- or leucine-supplemented diet
Rats from Experiment 2 received a diet supplemented with either alanine or leucine for 10 days. The day of the experiment they were overnight starved (PA Ala or PA Leu) or fed during 1 h either diet (PP Ala or PP Leu). Rates of total proteolysis and non-lysosomal Ca2+-independent proteolysis were measured in incubated epitrochlearis muscles as described in Methods. Values are means ±
s.e.m.
(vertical bars) for 9–10 animals and are expressed in nmol of Tyr (mg protein)−1 h−1. *P < 0.05 versus PA Leu as assessed by ANOVA.
Figure 5. mRNA levels for genes encoding components of the Ub–proteasome pathway in 8- and 22-month-old rats chronically fed an alanine- or leucine-supplemented diet
Eight- and 22-month-old rats from Experiment 2 received for 10 days a diet supplemented with either alanine (A) or leucine (B). The day of the experiment animals were overnight starved, extensor digitorum longus muscles were harvested, and Northern blots for Ub, the 14-kDa E2, and subunits C2 and X of the 20S proteasome were performed as described in Methods. Hybridization signals were quantified and normalized using the corresponding 18S rRNA signals to correct for uneven unloading. Both transcripts for Ub and the lower transcript 14-kDa E2 were quantified. Values are means ±
s.e.m.
(vertical bars) for n = 5–7 rats, and are expressed as percentage of 8-month-old rats fed the Ala-supplemented diet. Representative Northern blots are also shown. *P < 0.05 versus 8-month-old rats by the unpaired Student's t test.
Comment in
- A role for leucine in rejuvenating the anabolic effects of food in old rats.
Rennie MJ. Rennie MJ. J Physiol. 2005 Dec 1;569(Pt 2):357. doi: 10.1113/jphysiol.2005.099416. Epub 2005 Oct 6. J Physiol. 2005. PMID: 16210347 Free PMC article. No abstract available.
Similar articles
- Proteasome activity is altered in skeletal muscle tissue of tumour-bearing rats a leucine-rich diet.
Ventrucci G, Mello MA, Gomes-Marcondes MC. Ventrucci G, et al. Endocr Relat Cancer. 2004 Dec;11(4):887-95. doi: 10.1677/erc.1.00828. Endocr Relat Cancer. 2004. PMID: 15613461 - Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal.
Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Dardevet D, et al. J Nutr. 2002 Jan;132(1):95-100. doi: 10.1093/jn/132.1.95. J Nutr. 2002. PMID: 11773514 - Contrarily to whey and high protein diets, dietary free leucine supplementation cannot reverse the lack of recovery of muscle mass after prolonged immobilization during ageing.
Magne H, Savary-Auzeloux I, Migné C, Peyron MA, Combaret L, Rémond D, Dardevet D. Magne H, et al. J Physiol. 2012 Apr 15;590(8):2035-49. doi: 10.1113/jphysiol.2011.226266. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351629 Free PMC article. - The role of ubiquitin-proteasome-dependent proteolysis in the remodelling of skeletal muscle.
Taillandier D, Combaret L, Pouch MN, Samuels SE, Béchet D, Attaix D. Taillandier D, et al. Proc Nutr Soc. 2004 May;63(2):357-61. doi: 10.1079/PAR2004358. Proc Nutr Soc. 2004. PMID: 15294055 Review. - Skeletal muscle proteolysis in aging.
Combaret L, Dardevet D, Béchet D, Taillandier D, Mosoni L, Attaix D. Combaret L, et al. Curr Opin Clin Nutr Metab Care. 2009 Jan;12(1):37-41. doi: 10.1097/MCO.0b013e32831b9c31. Curr Opin Clin Nutr Metab Care. 2009. PMID: 19057185 Review.
Cited by
- Circulating Amino Acid Concentration after the Consumption of Pea or Whey Proteins in Young and Older Adults Affects Protein Synthesis in C2C12 Myotubes.
Salles J, Gueugneau M, Laleg K, Giraudet C, Sanchez P, Blot A, Richard R, Neveux N, Lefranc-Millot C, Perreau C, Guérin-Deremaux L, Boirie Y, Walrand S. Salles J, et al. Nutrients. 2024 Aug 27;16(17):2870. doi: 10.3390/nu16172870. Nutrients. 2024. PMID: 39275186 Free PMC article. - Curcumin-Added Whey Protein Positively Modulates Skeletal Muscle Inflammation and Oxidative Damage after Exhaustive Exercise.
Dias KA, da Conceição AR, Pereira SMS, Oliveira LA, da Silva Rodrigues JV, Dias RS, de Paula SO, Natali AJ, da Matta SLP, Gonçalves RV, Tako E, Martino HSD, Lucia CMD. Dias KA, et al. Nutrients. 2022 Nov 19;14(22):4905. doi: 10.3390/nu14224905. Nutrients. 2022. PMID: 36432591 Free PMC article. - Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer.
Jiménez-Alonso JJ, Guillén-Mancina E, Calderón-Montaño JM, Jiménez-González V, Díaz-Ortega P, Burgos-Morón E, López-Lázaro M. Jiménez-Alonso JJ, et al. Nutrients. 2022 Aug 17;14(16):3378. doi: 10.3390/nu14163378. Nutrients. 2022. PMID: 36014884 Free PMC article. - An umbrella review of systematic reviews of β-hydroxy-β-methyl butyrate supplementation in ageing and clinical practice.
Phillips SM, Lau KJ, D'Souza AC, Nunes EA. Phillips SM, et al. J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2265-2275. doi: 10.1002/jcsm.13030. Epub 2022 Jul 12. J Cachexia Sarcopenia Muscle. 2022. PMID: 35818771 Free PMC article. - Pea Proteins Have Anabolic Effects Comparable to Milk Proteins on Whole Body Protein Retention and Muscle Protein Metabolism in Old Rats.
Salles J, Guillet C, Le Bacquer O, Malnero-Fernandez C, Giraudet C, Patrac V, Berry A, Denis P, Pouyet C, Gueugneau M, Boirie Y, Jacobs H, Walrand S. Salles J, et al. Nutrients. 2021 Nov 25;13(12):4234. doi: 10.3390/nu13124234. Nutrients. 2021. PMID: 34959786 Free PMC article.
References
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000a;130:139–145. - PubMed
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000b;130:2413–2419. - PubMed
- Arnal M-A, Mosoni L, Boirie Y, Houlier M-L, Morin L, Verdier E, Ritz P, Antoine J-M, Prugnaud J, Beaufrère B, Patureau Mirand P. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. - PubMed
- Arnal M-A, Mosoni L, Dardevet D, Ribeyre M-C, Bayle G, Prugnaud J, Patureau Mirand P. Pulse protein feeding pattern restores stimulation of muscle protein synthesis during the feeding period in old rats. J Nutr. 2002;132:1002–1008. - PubMed
- Attaix D, Combaret L, Kee AJ, Taillandier D. Mechanisms of ubiquitination and proteasome-dependent proteolysis in skeletal muscle. In: Zempleni J, Daniel H, editors. Molecular Nutrition. Wallingford, Oxon, UK: CABI Publishing; 2003. pp. 219–235.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous