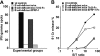TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo - PubMed (original) (raw)
TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo
Yaiza Diaz-de-Durana et al. Blood. 2006.
Abstract
We demonstrated that B-cell-dendritic cell (DC) interactions via transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI) and B-lymphocyte stimulator (BLyS) provide an early signal critical to generate adequate numbers of mature antigen presenting cells (APCs) to prime naive CD8(+) T cells (CTLs) in vivo. Evidence that B cells are required for efficient CTL generation in mice and that reconstitution with wild-type but not TACI-knockout B cells restored normal CTL responses support our conclusion. Moreover, low doses of a TACI fusion protein (TACI-Fc) that express the extracellular domain of TACI (amino acid [aa] 1-126) restored CTL priming in B-cell-deficient mice in vivo and induced DC maturation in vitro. In fact, following interactions with B cells, splenic DCs rapidly express the CD86 costimulatory molecule, to an extent comparable to the exposure to antigenic stimuli. BLyS(high) peptide-pulsed bone marrow-derived DCs, used as vaccines in vivo, cannot generate CTLs in B-cell-deficient and TACI-deficient mice, strongly supporting a need for B-cell-DC cooperation through TACI-BLyS during CTL first encounter with antigens in vivo.
Figures
Figure 1.
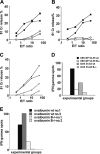
CTL priming in B-cell–deficient mice. Four CTL peptides of different MHC class I binding affinity were used as immunogens in B-cell–deficient mice and compared with wild-type C57BL/6 mice. Open symbols represent the cytolytic response in B-cell–deficient mice, and solid symbols represent responses in wild-type mice in all panels. Two experiments have been averaged (SD ≤ 6% lysis for all E/T ratios). (A) Specific lysis measured by 51Cr release using as immunogens 2 immunodominant and Th cell–independent viral sequences emulsified in IFA. (B) Immunization with the same CTL epitopes where T-cell help is provided by co-injection with a helper peptide, the sequence derived from the hepatitis B virus (HBV) HBc 128-140. (C) CTL induction following priming with 2 subdominant Th-dependent peptides co-injected with Th (HBc 128-140 for the CTL determinant LCMV NP 205-212 and OVA 323-336 for the CTL determinant OVA 55-62). (D) Representative ex vivo IFNγ measurement by the ELISPOT assay in response to the T helper–independent VSV NP 52-59 and T helper–dependent OVA 55-62 peptides, co-injected with the helper OVA 323-336 determinant (SD ≤ 2%). (E) Ex vivo IFNγ measurement by ELISPOT assay in CD4+ T-cell–depleted spleen cells in response to the whole OVA protein used as immunogen in CFA: representative CTL responses in 2 wild-type C57BL/6 mice and 2 B-cell–deficient mice from the same experiment.
Figure 2.
TACI-Fc treatment restores CTL expansion in B-cell–deficient mice. B-cell–deficient mice (2 per group) were pretreated with either TACI-Fc or B7RP-1–Fc fusion proteins (1 μg/mouse, intraperitoneally, 24 hours before and 48 hours after peptide priming) then immunized with the VSV NP 52-59 peptide in IFA. Untreated wild-type C57/BL6 mice were also immunized as a control. Mice were killed 7 days after immunization, their spleens were harvested and homogenized into a single-cell suspension, then analyzed ex vivo for peptide-specific IFNγ-producing cells by the ELISPOT assay (A) and for cytolytic activity following additional in vitro stimulation of primed splenocytes with peptide (B). An average of 2 experiments is shown with SD ranging from ≤ 3 (A) and ≤ 4 (B) at all E/T ratios tested.
Figure 3.
CD8+ T cells do not express a coreceptor for TACI. Total splenocytes from OT-1 mice were stimulated with the SIINFEKL peptide (0.1μg/mL) for 24 hours, and stained for FACS analysis with TACI-Fc followed by FITC-conjugated goat anti–human IgG. Human IgG (Ctrl IgG) was used as a control for nonspecific binding in the FACS analysis. Panel A shows the staining with TACI-Fc of unstimulated and peptide-stimulated CD8+ T cells; panel B shows CD69 expression in unstimulated and peptide-stimulated CD8+ T cells. Substantial binding of TACI-Fc to either unstimulated (A, filled histogram) or peptide-stimulated (A, empty histogram) CD8+ T cells was not detectable. The staining with TACI-Fc is in fact highly comparable with the Ctrl IgG control in both cell preparations. T-cell activation has been proven by the selective CD69 expression in the peptide-stimulated T cells (B, light gray histogram).
Figure 4.
BLyS up-regulation correlates with CD86 expression in maturing DCs exposed to a variety of antigenic stimuli or in B-cell cocultures. FACS-sorted splenic CD11c+ cells were cultured under different conditions to define a correlation between BLyS up-regulation and CD86 expression after exposure to a variety of antigenic stimuli or B-DC cocultures. Open bold histograms show the expression of BLyS in CD86+ gated cells compared with isotype controls (solid histograms). The culture conditions tested were: (A) GM-CSF alone; (B) 2.5 μg/mL LPS; (C) 5 × 106 necrotic EL-4 cells; (D) 5 × 106 apoptotic EL-4 cells; and (E) 5 × 106 splenic B cells.
Figure 5.
B-cell–DC cocultures induce CD11c+ cell maturation and increase cell division. Splenic CD11c+ cells were purified by FACS sorting and CFSE-labeled prior cocultures with B cells. CD11c+ cells alone have been used as control in these experiments. After 72 hours cells were collected and analyzed for cell division and expression of the CD86 maturation marker. Splenic CD11c+ were analyzed for purity after FACS sorting (A). SSC indicates side scatter. CFSE dilutions are shown in panel B, where B-cell–DC cocultures are represented by solid histograms and DCs alone by dotted histograms. Confirming previous results, CD86 was expressed in the large majority of the CD11c+ cells cocultured with B cells (C, top right quadrant). As expected, DC induced B-cell maturation through BLyS (C, top left quadrant).
Figure 6.
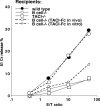
Requirement for B cells or TACI for DC priming in mice. VSV NP 52-59–specific cytolytic activity in mice primed with 2 × 106 mature, BLyShigh bone marrow–derived DCs pulsed with the VSV NP 52-59 peptide after terminal differentiation with TNFα. Three experiments, each containing 2 mice per group, have been averaged (SD ≤ 4 at all the E/T ratios studied). Three recipient mouse strains (wild-type C5BL7/6, and B-cell–deficient and TACI-deficient knockout strains) were used in these experiments, as indicated. To address the importance of TACI signaling in this priming protocol, 2 independent groups of B-cell–deficient mice have been treated either with TACI-Fc administered intraperitoneally 24 hours prior to and 48 hours after CTL priming, or TACI-Fc administered in vitro, the last 48 hours in DC cultures prior to peptide pulsing and in vivo transfer. The results of these experiments, shown with dotted lines, support the need for an ongoing TACI-BlyS signal during priming, as proven by the restored CTL induction in B-cell–deficient mice that received TACI-Fc in vivo but not in vitro (dotted lines).
Similar articles
- NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo.
Stober D, Jomantaite I, Schirmbeck R, Reimann J. Stober D, et al. J Immunol. 2003 Mar 1;170(5):2540-8. doi: 10.4049/jimmunol.170.5.2540. J Immunol. 2003. PMID: 12594280 - TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice.
Wang H, Marsters SA, Baker T, Chan B, Lee WP, Fu L, Tumas D, Yan M, Dixit VM, Ashkenazi A, Grewal IS. Wang H, et al. Nat Immunol. 2001 Jul;2(7):632-7. doi: 10.1038/89782. Nat Immunol. 2001. PMID: 11429548 - Therapeutic potential of CD8+ cytotoxic T lymphocytes in SLE.
Puliaeva I, Puliaev R, Via CS. Puliaeva I, et al. Autoimmun Rev. 2009 Jan;8(3):219-23. doi: 10.1016/j.autrev.2008.07.045. Epub 2008 Aug 24. Autoimmun Rev. 2009. PMID: 18725326 Free PMC article. Review. - Telitacicept, a novel humanized, recombinant TACI-Fc fusion protein, for the treatment of systemic lupus erythematosus.
Fan Y, Gao D, Zhang Z. Fan Y, et al. Drugs Today (Barc). 2022 Jan;58(1):23-32. doi: 10.1358/dot.2022.58.1.3352743. Drugs Today (Barc). 2022. PMID: 35107091 Review.
Cited by
- Human B cells induce dendritic cell maturation and favour Th2 polarization by inducing OX-40 ligand.
Maddur MS, Sharma M, Hegde P, Stephen-Victor E, Pulendran B, Kaveri SV, Bayry J. Maddur MS, et al. Nat Commun. 2014 Jun 9;5:4092. doi: 10.1038/ncomms5092. Nat Commun. 2014. PMID: 24910129 Free PMC article. - The receptor tyrosine kinase MerTK regulates dendritic cell production of BAFF.
Gohlke PR, Williams JC, Vilen BJ, Dillon SR, Tisch R, Matsushima GK. Gohlke PR, et al. Autoimmunity. 2009 Mar;42(3):183-97. doi: 10.1080/08916930802668586. Autoimmunity. 2009. PMID: 19301199 Free PMC article. - Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice.
Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Schmidt MR, et al. PLoS One. 2008 Sep 11;3(9):e3192. doi: 10.1371/journal.pone.0003192. PLoS One. 2008. PMID: 18784835 Free PMC article. - Effect of TACI signaling on humoral immunity and autoimmune diseases.
Zhang Y, Li J, Zhang YM, Zhang XM, Tao J. Zhang Y, et al. J Immunol Res. 2015;2015:247426. doi: 10.1155/2015/247426. Epub 2015 Mar 17. J Immunol Res. 2015. PMID: 25866827 Free PMC article. Review. - B cell maturation antigen deficiency exacerbates lymphoproliferation and autoimmunity in murine lupus.
Jiang C, Loo WM, Greenley EJ, Tung KS, Erickson LD. Jiang C, et al. J Immunol. 2011 Jun 1;186(11):6136-47. doi: 10.4049/jimmunol.1001931. Epub 2011 May 2. J Immunol. 2011. PMID: 21536804 Free PMC article.
References
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9: 271-296. - PubMed
- Banchereau J, Briere, F, Caux, C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18: 767-811. - PubMed
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106: 263-266. - PubMed
- Itano AA, Jenkins, MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4: 733-739. - PubMed
- Seder A, Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4: 835-842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous