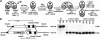Germ line transcripts are processed by a Dicer-like protein that is essential for developmentally programmed genome rearrangements of Tetrahymena thermophila - PubMed (original) (raw)
Germ line transcripts are processed by a Dicer-like protein that is essential for developmentally programmed genome rearrangements of Tetrahymena thermophila
Colin D Malone et al. Mol Cell Biol. 2005 Oct.
Abstract
Abundant approximately 28-nucleotide RNAs that are thought to direct histone H3 lysine 9 (H3K9) methylation and promote the elimination of nearly 15 Mbp of DNA from the developing somatic genome are generated during Tetrahymena thermophila conjugation. To identify the protein(s) that generates these small RNAs, we studied three Dicer-related genes encoded within the Tetrahymena genome, two that contain both RNase III and RNA helicase motifs, Dicer 1 (DCR1) and DCR2, and a third that lacks the helicase domain, Dicer-like 1 (DCL1). DCL1 is expressed upon the initiation of conjugation, and the protein localizes to meiotic micronuclei when bidirectional germ line transcription occurs and small RNAs begin to accumulate. Cells in which we disrupted the DCL1 gene (DeltaDCL1) grew normally and initiated conjugation as wild-type cells but arrested near the end of development and eventually died, unable to resume vegetative growth. These DeltaDCL1 cells failed to generate the abundant small RNAs but instead accumulated germ line-limited transcripts. Together, our findings demonstrate that these transcripts are the precursors of the small RNAs and that DCL1 performs RNA processing within the micronucleus. Postconjugation DeltaDCL1 cells die without eliminating the germ line-limited DNA sequences from their newly formed somatic macronuclei, a result that shows that this Dicer-related gene is required for programmed DNA rearrangements. Surprisingly, DeltaDCL1 cells were not deficient in overall H3K9 methylation, but this modification was not enriched on germ line-limited sequences as it is in wild-type cells, which clearly demonstrates that these small RNAs are essential for its targeting to specific loci.
Figures
FIG. 1.
Tetrahymena thermophila encodes three Dicer-like proteins. (A) Total predicted protein length from the Tetrahymena genome project is indicated at the right end of each schematic (see Materials and Methods for the locations of each within chromosomal scaffolds). Conserved domains identified by Pfam are indicated by the shaded or hatched boxes (see key). AA, amino acids; dsRNA, small RNA. (B, D, and E) Northern blot analysis was used to examine the expression of Dicer homologues at different life cycle stages. “E veg” refers to early-log-phase vegetative growth, and “L veg” refers to late-log/early-stationary-phase growth. The numbers above each lane denote the hour of mating when RNA was isolated. Arrowheads indicate transcript hybridization. The migration of RNA size markers (Promega) is presented on the left. (B) For DCL1 expression, a 1-day autoradiogram exposure is shown above a 3-day exposure (L.exp) (arrow with asterisk) that is used to reveal low-level expression at 6 h of conjugation and to highlight the absence of expression in vegetative cells. (C) RT-PCR analysis of RNA isolated from vegetative CU428 cells. The indicated amount of an in vitro-transcribed RNA was added to each 2-μg sample prior to reverse transcription to determine the sensitivity of the assay (1 fg = ∼1,000 transcripts). One-tenth (200-ng equivalents) was used in each PCR. ATU1 amplification was used to confirm cDNA synthesis. gDNA, genomic DNA. (D and E) DCR1 and DCR2 expression, respectively, was detected by 5-day exposure of blots to autoradiograph film. To compare loading between samples, each blot was stripped and rehybridized with an actin probe to reveal ACT1 expression, which is constitutive in vegetative (veg) and starved (stvd) cells but is initially down-regulated early during conjugation before returning to the vegetative level late in development (11 to 12 h).
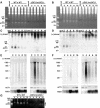
FIG. 2.
Germ line knockout of DCL1. (A) Knockout strategy. Biolistic transformation was employed to introduce the DCL1_-neo3 (n3) knockout construct into wild-type (wt) strains, and transformant progeny were selected in PM and subsequently in 6-methyl purine (MP) and then assorted to complete replacement in increasing concentrations of PM. The solid lines in the diagram indicate wild-type chromosomes; the lines with white arrows indicate knockout chromosomes. Transformants were converted to homozygosity by genomic exclusion crosses. Exconjugants were separated and assayed for the presence of the knockout construct. Strains homozygous for the construct in the micro- and macronucleus were used for phenotypic analyses, while those homozygous in the micronucleus and wild type in the macronucleus were transformed with the GFP-DCL1 construct and used in localization studies. Names are given below each strain with the macronuclear phenotype in parentheses and the micronuclear genotype in brackets. pm-r, paromomycin resistant. mp-r, 6-methyl purine resistant. (B) Southern blot analysis was used to verify the genotype Δ_DCL1 strains. Total genomic DNA was isolated, digested with HindIII, and hybridized with the DCL1 probe shown in the diagram to the left. The region replaced by the neo3 cassette (shaded arrow) relative to the conserved RNase III domains (solid boxes) and the lone intron (I) is depicted. The wild-type (WT) (closed triangle) and knockout (KO) (open triangle) HindIII fragments are 7.6 kb and 4.8 kb, respectively. Genomic DNA was analyzed from the two original wild-type strains, five somatic Δ_DCL1_ strains that are heterozygous in the micronucleus (n3/+), and four somatic DCL1 knockouts that are homozygous in the micronucleus for Δ_DCL1_ (n3/n3) and which were derived from the heterozygotes shown.
FIG. 3.
Δ_DCL1_ strains arrest late in conjugation. (A) RT-PCR was used to confirm that DCL1 was not expressed in knockouts. Total RNA isolated at 2 and 4 h of mating was converted to cDNA to be used as a template for PCR amplification with DCL1_-specific primers (Table 1), which are indicated as arrows in the knockout construct diagram (Fig. 2B). Identical reactions with ATU primers and wild-type (WT) genomic DNA (gDNA) served as positive controls for cDNA conversion and PCR amplification, respectively. Omission of reverse transcriptase (RT) controlled for the possibility of contaminating DNA in the reactions. (B) A diagram of the nuclear configuration diagnostic of individual stages is presented. The progression of wild-type (black bars) and Δ_DCL1 (gray bars) cells at individual time points after mixing was assessed by fluorescence microscopy of DAPI-staining cells. Numbers indicate the percentage of cell pairs at that stage of conjugation. (C) DAPI-stained cells showing the end point of development reached by wild-type and Δ_DCL1_ cells at 32.5 h of conjugation. White arrows point to micronuclei.
FIG. 4.
Conjugating Δ_DCL1_ strains exhibit loss of small RNA production and germ line transcript accumulation. RNA isolated at 2-h intervals from the start of conjugation was separated by electrophoresis on either 15% polyacrylamide-urea gels (A to D) or 1.2% agarose-formaldehyde gels (E and F), ethidium bromide stained (A and B) or transferred to membranes (C to F), and hybridized to plus-strand (+)- and minus-strand (−)-detecting M-element riboprobes as indicated. The migration of oligonucleotide (A to D) or RNA size standards (E and F) are indicated to the left of each panel. (A to D) RNA species of 28 to 30 nt (arrowhead) were observed throughout conjugation of wild-type (WT) cells but were undetected in starved (stvd) cells or Δ_DCL1_ conjugating strains. (E and F) Northern blot analysis of Δ_DCLl_ strains shows an accumulation of M-element bidirectional transcripts. Each filter was rehybridized with ACT1 and PDD1 probes for comparison of loading between samples and is shown below the corresponding panel. (G) Stained polyacrylamide gel of RNA isolated from wild-type or Δ_DCL1_ cells that reveals smaller species of short RNAs (open arrowhead) migrating below the position of the abundant ∼28-nt species (solid arrowhead).
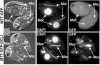
FIG. 5.
The DCL1 protein is localized to meiotic micronuclei. Tetrahymena transformed with pIGF-1, which contains GFP only (top panels) or a GFP-DCL1 construct (bottom panels) was mated to nontransformed wild-type or Δ_DCL1_ cells, and expression of the fusion protein was induced by the addition of CdCl2 upon mixing of cells. Differential interference contrast (DIC) light microscopy of single pairs is displayed adjacent to fluorescence imaging of DAPI-stained DNA with and the localization of GFP or the GFP-DCL1 fusion protein. The DCL1 protein is observed exclusively in the extrachromosomal space in the elongated, meiotic (prophase) micronuclei (labeled as Mic). The location of the macronucleus (Mac) is also indicated. The mating partner with the brighter GFP fluorescence signal is likely the transformant expressing the GFP fusion that typically shows greater fluorescence despite extensive cytoplasmic exchange within the pair. Background fluorescence apparent in vacuoles is common in DAPI and GFP fluorescence in live Tetrahymena and accounts for the cytoplasmic signal observed.
FIG. 6.
DCL1 knockouts fail to excise micronucleus-limited DNA. (A) Diagram of the micronuclear (mic) and macronuclear ¯ L/M/R and CaM loci. IESs are indicated as open boxes, and macronucleus-destined sequences are indicated as shaded boxes. The L/M/R locus contains three IESs named the L (left), M (middle), and R (right) deletion elements. The M element has two alternative, leftward deletion boundaries that generate two rearranged forms of this loci at nearly equally frequencies. The genomic region upstream of the CaM gene contains a 1.4-kbp IES. The locations of the M3 and CaM probes used in Southern blot hybridization are shown (10). (B to D) Southern blot hybridization of DNA from postconjugative wild-type and Δ_DCL1_ cells was used to assess IES rearrangement efficiency. For each blot, EcoRI-digested genomic DNA was fractionated, and specific loci were detected with (B) M3-, (C) CaM-, or (D) Tt2512-radiolabeled probes. The stained gel prior to blotting is shown for comparison of loading. Membranes were stripped and probed with the ATU1 probe (not shown) to measure relative quantities of DNA loaded in each lane, which are reported at the bottom of each lane, with the Δ_DCL1_ lanes set to 1 for ease of comparison. (E) Quantification of the rearranged (R) and unrearranged (U) forms in wild-type cells compared to Δ_DCL1_ cells for the L/M/R and CaM loci and relative hybridization intensities (adjusted to ATU1 hybridization) of EcoRI fragment (Frag) 1 (2.1 kb) and fragment 2 (1.7 kb) in the Tt2512 region in wild-type compared to DCL1 knockout cells. The measured Tt2512 hybridization for wild-type cells was arbitrarily set to 1.
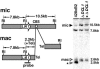
FIG. 7.
Chromosome breakage does not occur in DCL1 knockouts. The diagram shows the left end of macronuclear chromosomal scaffold CH445662, which contains the LIA1 gene within 2.5 kbp from the telomere (Tel), and the deduced ∼18-kbp region of the micronuclear (mic) chromosome from which it is derived. The predicted location of the chromosomal breakage sequence (CBS) (white oval) is depicted as well as the relevant EcoRI (RI) restriction sites used for the Southern blot analysis of genomic DNA from postconjugative wild-type and Δ_DCL1_ cells used to assess chromosome breakage. The probe spans the central EcoRI site and detects a 7.8-kbp fragment common to both nuclei, which can be used to compare amounts of DNA loaded between each lane, and either the ∼10.5-kbp micronucleus-specific fragment (solid arrowhead) or a 2.5- to 2.6-kbp macronucleus-specific fragment (2.2 kbp of unique sequence plus 300 to 400 bp of telomeric DNA) (open arrowheads). The shorter macronuclear fragments marked by the asterisk appear only in wild-type samples and are likely derived from new chromosomal breakage and telomere addition in developing macronuclei, while the larger fragments are presumed to be derived from the macronuclei of unmated cells with, on average, longer telomeres.
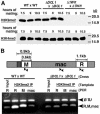
FIG. 8.
DCL1 is not required for H3K9 methylation. (A) Total cell protein extracts isolated from conjugating wild-type (WT), Δ_DCL1_, or Δ_TWI1_ cells at the indicated times after mixing were fractionated by SDS-polyacrylamide gel electrophoresis for Western blot analysis. The overall level of modified histone H3 was assessed with antibodies detecting histone H3K9me2 (dimethyl) (top) or histone H3K4me3 (trimethyl) (bottom). The positions of protein molecular mass standards (in kilodaltons [kD]) are given on the right. (B) Representative PCR results after chromatin immunoprecipitation with anti-H3K9me2-specific antibodies from 9-h mating Tetrahymena cells. PCR products of specific amplified fragments of the R or M element or the intervening macronucleus-retained sequence were separated by 1.6% agarose gel electrophoresis and stained with ethidium bromide. The locations of amplification primers are shown in the diagram of the analyzed genomic region as arrowheads. Input designates that the DNA template was recovered from chromatin preparations prior to immunoprecipitation (only amplification of input using R-element primers is shown; the upper band corresponds to amplification of a quantification control from the BTU1 locus, and the lower band corresponds to the IES or macronucleus-retained locus [indicated above each lane]).
Similar articles
- Histone H3 lysine 9 methylation is required for DNA elimination in developing macronuclei in Tetrahymena.
Liu Y, Mochizuki K, Gorovsky MA. Liu Y, et al. Proc Natl Acad Sci U S A. 2004 Feb 10;101(6):1679-84. doi: 10.1073/pnas.0305421101. Epub 2004 Jan 30. Proc Natl Acad Sci U S A. 2004. PMID: 14755052 Free PMC article. - Centromeric histone H3 is essential for vegetative cell division and for DNA elimination during conjugation in Tetrahymena thermophila.
Cui B, Gorovsky MA. Cui B, et al. Mol Cell Biol. 2006 Jun;26(12):4499-510. doi: 10.1128/MCB.00079-06. Mol Cell Biol. 2006. PMID: 16738316 Free PMC article. - Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in tetrahymena.
Mochizuki K, Fine NA, Fujisawa T, Gorovsky MA. Mochizuki K, et al. Cell. 2002 Sep 20;110(6):689-99. doi: 10.1016/s0092-8674(02)00909-1. Cell. 2002. PMID: 12297043 - Small RNAs in genome rearrangement in Tetrahymena.
Mochizuki K, Gorovsky MA. Mochizuki K, et al. Curr Opin Genet Dev. 2004 Apr;14(2):181-7. doi: 10.1016/j.gde.2004.01.004. Curr Opin Genet Dev. 2004. PMID: 15196465 Review. - Dynamic nuclear reorganization during genome remodeling of Tetrahymena.
Chalker DL. Chalker DL. Biochim Biophys Acta. 2008 Nov;1783(11):2130-6. doi: 10.1016/j.bbamcr.2008.07.012. Epub 2008 Jul 28. Biochim Biophys Acta. 2008. PMID: 18706458 Free PMC article. Review.
Cited by
- A SUMO E3 ligase promotes long non-coding RNA transcription to regulate small RNA-directed DNA elimination.
Shehzada S, Noto T, Saksouk J, Mochizuki K. Shehzada S, et al. Elife. 2024 Jan 10;13:e95337. doi: 10.7554/eLife.95337. Elife. 2024. PMID: 38197489 Free PMC article. - Programmed chromosome fragmentation in ciliated protozoa: multiple means to chromosome ends.
Bétermier M, Klobutcher LA, Orias E. Bétermier M, et al. Microbiol Mol Biol Rev. 2023 Dec 20;87(4):e0018422. doi: 10.1128/mmbr.00184-22. Epub 2023 Nov 27. Microbiol Mol Biol Rev. 2023. PMID: 38009915 Review. - The Tetrahymena bcd1 mutant implicates endosome trafficking in ciliate, cortical pattern formation.
Cole ES, Maier W, Joachimiak E, Jiang YY, Lee C, Collet E, Chmelik C, Romero DP, Chalker D, Alli NK, Ruedlin TM, Ozzello C, Gaertig J. Cole ES, et al. Mol Biol Cell. 2023 Jul 1;34(8):ar82. doi: 10.1091/mbc.E22-11-0501. Epub 2023 May 10. Mol Biol Cell. 2023. PMID: 37163326 Free PMC article. - MITE infestation accommodated by genome editing in the germline genome of the ciliate Blepharisma.
Seah BKB, Singh M, Emmerich C, Singh A, Woehle C, Huettel B, Byerly A, Stover NA, Sugiura M, Harumoto T, Swart EC. Seah BKB, et al. Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2213985120. doi: 10.1073/pnas.2213985120. Epub 2023 Jan 20. Proc Natl Acad Sci U S A. 2023. PMID: 36669106 Free PMC article. - PIWI-Directed DNA Elimination for Tetrahymena Genetics.
Shehzada S, Mochizuki K. Shehzada S, et al. Methods Mol Biol. 2022;2509:53-68. doi: 10.1007/978-1-0716-2380-0_3. Methods Mol Biol. 2022. PMID: 35796956
References
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1990. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- Bernstein, E., A. A. Caudy, S. M. Hammond, and G. J. Hannon. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-366. - PubMed
- Bruns, P. J., and D. Cassidy-Hanley. 2000. Biolistic transformation of macro- and micronuclei. Methods Cell Biol. 62:501-512. - PubMed
- Bruns, P. J., and D. Cassidy-Hanley. 2000. Methods for genetic analysis. Methods Cell Biol. 62:229-240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous