Pharmacological manipulation of Bcl-2 family members to control cell death - PubMed (original) (raw)
Pharmacological manipulation of Bcl-2 family members to control cell death
Anthony Letai. J Clin Invest. 2005 Oct.
Abstract
The commitment to programmed cell death involves complex interactions among pro- and antiapoptotic members of the Bcl-2 family of proteins. The physiological result of a decision by these proteins to undergo cell death is permeabilization of the mitochondrial outer membrane. Pharmacologic manipulation of proteins in this family appears both feasible and efficacious, whether the goal is decreased cell death, as in ischemia of the myocardium or brain, or increased cell death, as in cancer.
Figures
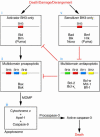
Figure 1
A model of Bcl-2 family member control over programmed cell death. In response to myriad death, damage, or derangement signals, BH3-only family members are activated (i). Activator BH3-only proteins interact with multidomain proapoptotic Bax and/or Bak (Bax/Bak), inducing their oligomerization (ii) and thus resulting in MOMP, release of cytochrome c, apoptosome formation, and caspase activation (iii). Bcl-2 and other multidomain antiapoptotic proteins interrupt the death signal by binding and sequestering activator BH3-only family members, and perhaps also Bax/Bak (iv). Bcl-2 antiapoptotic function may be antagonized by the competitive displacement of activator BH3-only molecules by sensitizer BH3-only proteins (v).
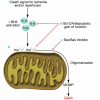
Figure 2
Model of interventions to reduce ischemic and ischemia/reperfusion injury. (i and ii) Following ischemia or ischemia/reperfusion, cell death signals are initiated (i) and conducted to the intrinsic pathway via activated BH3-only family members (ii). (iii) These BH3 family members interact with Bax/Bak, inducing oligomerization, MOMP, and commitment to cell death. (iv) Treatment with a viral vector expressing Bcl-2 or other antiapoptotic gain of function might prevent BH3-only activation of Bax/Bak and/or oligomerization of Bax/Bak. (v) Treatment with a Bax/Bak inhibitor might prevent Bax/Bak induction of MOMP.
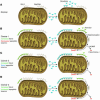
Figure 3
Model for targeting cancer cells with sensitizer BH3 mimetics. (A) Mitochondrion from a normal cell has some Bax/Bak and Bcl-2. Bcl-2 is unoccupied; normal cell behavior is provoking no death signals. (B–D) Mitochondria from cancer cells have equal Bax/Bak and overexpress Bcl-2 in this model. Antiapoptotic reserve is defined as the number of unoccupied antiapoptotic Bcl-2 family member binding pockets per cell. Compared with normal mitochondria, those that overexpress Bcl-2 may provide decreased (B), equal (C), or increased (D) antiapoptotic reserve. Because of genomic instability, oncogene activation, cell cycle checkpoint violation, or perhaps cancer-specific response to cytotoxic chemotherapy, activator BH3 domains have been triggered and are sequestered by Bcl-2. After exposure to a sensitizer BH3 mimetic (a protein, peptide, or small molecule), activator BH3 domains are displaced from cancer cells, but not normal cells, activating Bax/Bak and allowing selective cancer cell killing, perhaps even as a single agent. It can be seen why sensitizer mimetics might offer a greater therapeutic window than an activator, as an activator molecule would provide selective killing only at low doses and only for cancer cells in condition 1 (Cancer 1). At higher doses, or if the cancer cells were in condition 2 or 3, there would be killing of normal and cancer cells. It is unclear whether activator- or sensitizer-type BH3-only family members predominate in the response to conventional chemotherapy agents, and it is likely that a mixture is present. These models also speculate why certain cancers, such as follicular lymphoma and chronic lymphocytic leukemia, despite expressing higher levels of Bcl-2, are more prone to apoptosis than normal cells after DNA-damaging chemotherapy.
Similar articles
- Flow Cytometry-Based Detection and Analysis of BCL-2 Family Proteins and Mitochondrial Outer Membrane Permeabilization (MOMP).
Ludwig LM, Maxcy KL, LaBelle JL. Ludwig LM, et al. Methods Mol Biol. 2019;1877:77-91. doi: 10.1007/978-1-4939-8861-7_5. Methods Mol Biol. 2019. PMID: 30535999 Free PMC article. - Neurotoxic nitric oxide rapidly depolarizes and permeabilizes mitochondria by dynamically opening the mitochondrial transition pore.
Kindler DD, Thiffault C, Solenski NJ, Dennis J, Kostecki V, Jenkins R, Keeney PM, Bennett JP Jr. Kindler DD, et al. Mol Cell Neurosci. 2003 Aug;23(4):559-73. doi: 10.1016/s1044-7431(03)00074-5. Mol Cell Neurosci. 2003. PMID: 12932437 - Regulation of Apoptosis by the Bcl-2 Family of Proteins: Field on a Brink.
Ladokhin AS. Ladokhin AS. Cells. 2020 Sep 18;9(9):2121. doi: 10.3390/cells9092121. Cells. 2020. PMID: 32961920 Free PMC article. - Cellular neuroprotective mechanisms in cerebral ischemia: Bcl-2 family proteins and protection of mitochondrial function.
Ouyang YB, Giffard RG. Ouyang YB, et al. Cell Calcium. 2004 Sep-Oct;36(3-4):303-11. doi: 10.1016/j.ceca.2004.02.015. Cell Calcium. 2004. PMID: 15261486 Review. - Larger than life: Mitochondria and the Bcl-2 family.
Skommer J, Wlodkowic D, Deptala A. Skommer J, et al. Leuk Res. 2007 Mar;31(3):277-86. doi: 10.1016/j.leukres.2006.06.027. Epub 2006 Sep 5. Leuk Res. 2007. PMID: 16911824 Review.
Cited by
- Lipid raft: A floating island of death or survival.
George KS, Wu S. George KS, et al. Toxicol Appl Pharmacol. 2012 Mar 15;259(3):311-9. doi: 10.1016/j.taap.2012.01.007. Epub 2012 Jan 24. Toxicol Appl Pharmacol. 2012. PMID: 22289360 Free PMC article. Review. - The mitochondrial death pathway: a promising therapeutic target in diseases.
Gupta S, Kass GE, Szegezdi E, Joseph B. Gupta S, et al. J Cell Mol Med. 2009 Jun;13(6):1004-33. doi: 10.1111/j.1582-4934.2009.00697.x. Epub 2009 Feb 9. J Cell Mol Med. 2009. PMID: 19220575 Free PMC article. Review. - Dual mechanisms of sHA 14-1 in inducing cell death through endoplasmic reticulum and mitochondria.
Hermanson D, Addo SN, Bajer AA, Marchant JS, Das SG, Srinivasan B, Al-Mousa F, Michelangeli F, Thomas DD, Lebien TW, Xing C. Hermanson D, et al. Mol Pharmacol. 2009 Sep;76(3):667-78. doi: 10.1124/mol.109.055830. Epub 2009 Jun 26. Mol Pharmacol. 2009. PMID: 19561125 Free PMC article. - Ulinastatin inhibits renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction.
Zhang QF. Zhang QF. Mol Med Rep. 2017 Dec;16(6):8916-8922. doi: 10.3892/mmr.2017.7692. Epub 2017 Oct 3. Mol Med Rep. 2017. PMID: 28990075 Free PMC article. - Pharmacological manipulation of cell death: clinical applications in sight?
Green DR, Kroemer G. Green DR, et al. J Clin Invest. 2005 Oct;115(10):2610-7. doi: 10.1172/JCI26321. J Clin Invest. 2005. PMID: 16200193 Free PMC article. Review.
References
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. - PubMed
- Bakhshi A, et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. - PubMed
- McDonnell TJ, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. - PubMed
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases