Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt - PubMed (original) (raw)
Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt
Takahiro Kato et al. J Clin Invest. 2005 Oct.
Abstract
This study delineates a mechanism for antiapoptotic signaling initiated by atrial natriuretic peptide (ANP) stimulation leading to elevation of cGMP levels and subsequent nuclear accumulation of Akt kinase associated with zyxin, a cytoskeletal LIM-domain protein. Nuclear targeting of zyxin induces resistance to cell death coincident with nuclear accumulation of activated Akt. Nuclear translocation of zyxin triggered by cGMP also promotes nuclear Akt accumulation. Additional supportive evidence for nuclear accumulation of zyxin-enhancing cardiomyocyte survival includes the following: (a) promotion of zyxin nuclear localization by cardioprotective stimuli; (b) zyxin association with phospho-Akt473 induced by cardioprotective stimuli; and (c) recruitment of zyxin to the nucleus by activated nuclear-targeted Akt as well as recruitment of Akt by nuclear-targeted zyxin. Nuclear accumulation of zyxin requires both Akt activation and nuclear localization. Potentiation of cell survival is sensitive to stimulation intensity with high-level induction by ANP or cGMP signaling leading to apoptotic cell death rather than enhancing resistance to apoptotic stimuli. Myocardial nuclear accumulation of zyxin and Akt responds similarly in vivo following treatment of mice with ANP or cGMP. Thus, zyxin and activated Akt participate in a cGMP-dependent signaling cascade leading from ANP receptors to nuclear accumulation of both molecules. Nuclear accumulation of zyxin and activated Akt may represent a fundamental mechanism that facilitates nuclear-signal transduction and potentiates cell survival.
Figures
Figure 1
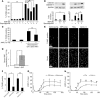
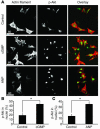
ANP exposure exerts concentration-dependent antiapoptotic effects upon cardiomyocytes and is protective in vivo. (A) Cardiomyocytes were plated onto chamber slides and preincubated with or without ANP (concentration range from 10–10.5 M to 10–6 M as indicated for 1 hour) followed by 2 hours of vehicle only (–) or staurosporine treatment (+; 1 μM). Apoptosis was assessed by TUNEL assay. (B) Apoptotic signaling in cultured cardiomyocytes evaluated by cleaved caspase-3. Quantitation of caspase-3 immunoblot was performed by densitometric analysis using GAPDH as a loading standard to correct for slight differences in protein loading. ANP treatment protocol was the same as in A. (C) Cardiomyocytes were treated with ANP for 24 hours prior to 2 hours apoptotic stimulus with staurosporine. Apoptosis was assessed by TUNEL assay. (D) Circulating serum levels of ANP from mice implanted with osmotic pumps as determined by ELISA assay (see Methods; n = 4 for each group). (E–H) Analyses of hearts from mice implanted with osmotic pumps (saline control versus ANP treated) or genetically engineered to express nuclear-targeted zyxin (nontransgenic [NTg] control versus nuclear-targeted zyxin [zyxin-n.t.]) as described in Methods. (E) Representative confocal micrographs of TUNEL assays performed on myocardial sections of hearts subjected to ischemia/reperfusion damage. Scale bars: 10 μm. (F) Quantitation of TUNEL-positive nuclei from myocardial sections of mice as indicated. (G–H) Recovery of hemodynamic function during reperfusion phase for mouse groups receiving osmotic pump implants (G) or genetically engineered to express nuclear targeted zyxin (H). *P < 0.05 or **P < 0.01 for each indicated comparison in A–H. n = 3 for experiments in A–C; n = 4 for experiments in D–H. LVDP, left ventricular developed pressure.
Figure 2
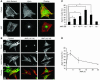
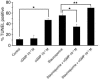
ANP induces nuclear accumulation of zyxin at antiapoptotic concentration. Immunolabeling (A and B) and quantitation of nuclear labeling (C and D) in cultured cardiomyocytes treated with ANP (10–9 for 1 hour for A, B, and D; 10–6 M for 1 hour also shown for B). Immunolabeling at high (A) and low (B) magnification shows zyxin (green in overlay) predominantly associated with myofibrillar striations and focal adhesion regions in periphery of untreated cells (control). 10–9 M ANP treatment resulted in redistribution and nuclear accumulation of zyxin whereas nuclear accumulation did not occur at 10–6 M ANP. Myofibrillar organization was observed with phalloidin to label actin filaments (red in overlay). Bar measurements in micrographs represent distance in μm. Quantitation (C) shows dosage-dependent nuclear accumulation of zyxin peak at 10–9 M ANP 2 hours after treatment. Time course of nuclear zyxin accumulation (D) shows peak level within hours after treatment and return to normal level within 24 hours. n = 3 for all experiments. *P < 0.01.
Figure 3
Nuclear accumulation of zyxin is antiapoptotic. TUNEL assay (A) of cultured cardiomyocytes infected with adenoviruses expressing GFP, full-length zyxin (zyxin-w.t.), or nuclear-targeted zyxin for 2 hours followed by treatment with 1 μmol/l staurosporine to induce apoptosis. Results shown were derived from 3 separate experiments. (B) Apoptosis in cultured cardiomyocyte evaluated by DNA laddering. Adenoviruses expressing β-gal or zyxin as wild-type, GFP-conjugated full length (GFP), residues 1-322 (N-term), residues 349–542 (C-term), or NES-deleted residues 322–331 (NES). Apoptotic stimulation was initiated by overexpression of pyk2 kinase as previously described (35). Typical result is shown of 3 separate repetitions for the laddering experiment. (C) TUNEL assay of cultured cardiomyocytes shows protective effect of nuclear-targeted zyxin accumulation in response to apoptotic challenge by ANP (10–6 M) but lack of protection by ANP (10–9 M) in response to apoptotic challenge by full-length wild-type zyxin accumulation. Adenoviral vectors were used for expression of zyxin constructs with GFP expression shown as a control for effects of ANP treatments. *P < 0.01; **P < 0.05.
Figure 4
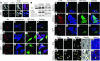
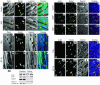
Nuclear accumulation of activated Akt kinase in response to ANP treatment or expression of nuclear-targeted zyxin. Cultured cardiomyocytes (A–Z) or myocardial sections (AA–JJ) showing nuclear accumulation of Akt. Confocal micrographs (A) and immunoblot analysis (B) of myocytes treated with vehicle (control) or ANP (10–6 M) show nuclear accumulation of both zyxin and phospho-Akt473 (p-Akt) in response to ANP exposure. Overlay channels in A correspond to zyxin (red), phospho-Akt (green), and actin filaments to show myofibril organization (blue). In B, immunoblots for GAPDH, histone H3, and connexin 43 demonstrate subcellular fractionation of the cytosolic, nuclear, and membrane fractions, respectively. Also shown are confocal micrographs of cardiomyocytes labeled with antibodies (shown in red) to either total Akt (C–N) or phospho-Akt473 (O–Z). Cells were infected with adenovirus (shown in green) expressing GFP (K–N and W–Z), nuclear-targeted zyxin (C–F and O–R), or full-length zyxin (G–J and S–V). Myofibrillar organization was observed with phalloidin to label actin filaments (shown in blue). (C–N) Akt immunoreactivity was mainly observed in cytoplasm in cultures expressing full-length zyxin or GFP but accumulated in the nucleus following expression of nuclear-targeted zyxin virus. (O–Z) Phospho-Akt473 immunoreactivity was observed at low levels in cells expressing either full-length zyxin or GFP but accumulated in the nucleus following expression of nuclear-targeted zyxin. Myocardial sections (AA–JJ) show nuclear accumulation of phospho-Akt473 in transgenic mice expressing cardiac-specific nuclear-targeted zyxin (zyxin-n.t. Tg) but not in nontransgenic control samples. Arrows indicate nuclei positive for both zyxin and phospho-Akt473. Transgenic zyxin was visualized using antibody to myc-tag (tag) and desmin to show myofibrillar structure. Overlay colors correspond to phospho-Akt473 (red), myc-tag (green), nuclei (pink), and desmin (blue) with coincidence of red and blue labeling appearing yellow. Scale bars: 20 μm (A and AA–JJ); 30 μm (C–Z).
Figure 5
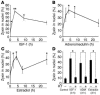
Nuclear accumulation of zyxin is mediated by cGMP-dependent signaling. Time course of zyxin nuclear accumulation in cultured cardiomyocytes treated with cGMP (10–4 M). (A) Confocal microscopy shows distribution of zyxin (green in overlay) and myofibrils (red in overlay) following exposure to cGMP. Cells were labeled with phalloidin (actin filament) to reveal sarcomeric organization. Scale bars: 30 μm. (B) Time course of nuclear zyxin accumulation shows peak level within minutes after treatment and return to normal level within 3 hours. (C) Activation of PKA or PKC signaling did not induce zyxin nuclear translocation. Bar graph shows the ratio of zyxin nuclear translocation with cGMP (10–4 M), PMA (10 ng/ml), or forskolin (10–5 M) treatment for 1 hour as assessed by confocal microscopy. (D) Participation of ANP or PKG in nuclear translocation of zyxin was demonstrated by use of HS142-1 (ANP receptor inhibitor; 10 μg/ml) or KT5823 (PKG inhibitor; 5 × 10–6 M). Bar graph shows the ratio of zyxin nuclear translocation with or without inhibitor pretreatment followed by stimulation with cGMP (10–4 M) or ANP (10–9 M). n = 3 for all experiments.
Figure 6
Elevation of cGMP induces rapid nuclear accumulation of activated Akt kinase. cGMP and ANP increased the expression level of phospho-Akt in the nuclear fraction. Cells were labeled with phalloidin (A, left side) to reveal sarcomeric organization. Confocal microscopy shows the nuclear accumulation of phospho-Akt increase in the cardiomyocyte with cGMP (10–4 M) or ANP (10–9 M) treatment (A, middle). Actin filament (red) and zyxin (green) are depicted in overlay panels (A, right side). Scale bars, 60 μm. (B and C) Bar graph shows the ratio of the nuclear accumulation of phospho-Akt with cGMP (10–4 M) or ANP (10–9 M) treatment obtained from confocal microscopic analysis. n = 3. *P < 0.05.
Figure 7
Antiapoptotic effect of cGMP exposure varies with concentration. TUNEL assay shows effect of cGMP treatment upon apoptosis induced by staurosporine (1 μM) in cultured cardiomyocytes at low (10–4) or high (10–2) concentration. *P < 0.01; **P < 0.05.
Figure 8
Cardioprotective stimuli induce nuclear accumulation of zyxin that depends upon PKG activity. Time course of zyxin nuclear accumulation following treatment of cultured cardiomyocytes with various antiapoptotic agents as indicated below the x axis of each graph is shown. Line graphs show the percentage of cells possessing nuclear localized zyxin following treatment with IGF-1 (A; 10–7 M), adrenomedullin (B; 10–7 M), and estradiol (C; 10–7 M) as assessed by confocal microscopy. Nuclear accumulation induced by any of the stimuli in A–C was inhibited by pretreatment of the cultures with KT5823 (PKG inhibitor; 5 × 10–6 M) as shown in D. n = 3 for all experiments. *P < 0.05; **P < 0.01.
Figure 9
ANP induces nuclear accumulation of zyxin and phospho-Akt in the myocardium. Myocardial sections (A–JJ) and immunoblot (KK) demonstrating nuclear accumulation of zyxin or phospho-Akt473. All ANP treatments were performed for 24 hours using an implanted osmotic pump with 200 μl of 10–4 M ANP. For all cGMP treatments, agonist at a concentration of 10–2 M was administered intravenously in 100 μl volume, and hearts were removed 5 hours later. (A–P) Control (A–D and I–L) or experimental mice (E–H and M–P) labeled with antibody to zyxin (A, E, I, and M; green in overlay). Cardiomyocytes were identified with antibodies to α-actinin (C and G) or desmin (K and O) (both blue in overlay). Nuclei were stained with TOPRO (B, F, J, and N; red in overlay). Nuclear accumulation of zyxin was observed either following ANP treatment or in transgenic mice expressing cardiac-specific GC (GC Tg). (Q–JJ) Control (Q–T and CC–FF) or experimental (U–BB and GG–JJ) mouse hearts labeled with antibody to phospho-Akt473 (Q, U, Y, CC, GG; yellow in overlay in panels X, BB, and JJ is due to coincident nuclear staining). Cardiomyocytes were identified with staining for desmin (S, W, AA, EE, and II; blue in overlays), and nuclei were identified by staining with TOPRO (R, V, Z, DD, and HH; red in overlays). Nuclear accumulation of phospho-Akt473 was observed following cGMP treatment (U–X; 5 hr intravenous), ANP treatment (Y–BB), andn in the cardiac-specific GC Tg mouse hearts (GG–JJ). (KK) Immunoblot of cardiac protein lysates from fractions of control or ANP-treated mice partitioned into nuclear and cytoplasmic/membrane fractions. Enrichment of zyxin or phospho-Akt473 was observed in samples from ANP-treated mice. Enrichment for nuclei was demonstrated by partitioning of GAPDH (cytoplasmic), histone H3 (nuclear), and connexin 43 (membrane associated) to appropriate fractions. Appropriate mobilities of bands on blots were confirmed using molecular weight standards in combination with labeling of unfractionated lysate (not shown). Scale bars: 10 μm.
Figure 10
Cardioprotective stimuli induce association of activated Akt with zyxin. Lysates (300 mg total protein) prepared from cultured cardiomyocytes treated with vehicle (control) or ANP (10–9 M for 30 or 60 minutes) were immunoprecipitated with anti–phospho-Akt473 antibody, and presence of zyxin was detected by subsequent immunoblotting with anti-zyxin antibody. Whole cell lysate prior to immunoprecipitation is shown to indicate presence of zyxin (positive control). Immunolabeling with phospho-Akt473 antibody confirmed immunoprecipitation of protein (p-Akt). Immunoprecipitation with irrelevant antibody to anti-p16ink4a is shown as a negative control. WB, Western blot.
Figure 11
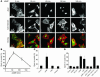
Nuclear-targeting of activated Akt induces nuclear accumulation of zyxin, and inhibition of Akt activation impairs nuclear accumulation of zyxin. Consequences of inhibiting Akt activation for zyxin nuclear localization (A and B) and Akt-mediated effects upon localization of zyxin in vivo (C–L). (A) Confocal micrographs of cultured cardiomyocytes treated with ANP alone or ANP following prior inhibitor treatments of LY294002 (LY) or wortmannin (WM). Distribution of zyxin (green in overlay) was influenced in all 3 groups, but inhibitor treatments prevented nuclear accumulation of zyxin. Nuclei were labeled with TOPRO (blue in overlay), and phalloidin shows actin filament structure (red). Scale bar: 10 μm (all scans in A at identical scale). (B) Bar graph shows the percentage of cells possessing nuclear localized zyxin following treatment with ANP (10–9 M) as assessed by confocal microscopy. Treatment groups from left to right are: no treatment with pharmacologic inhibitors; ANP treatment with or without pharmacologic inhibitors; adenoviral infections without ANP treatment; adenoviral infections with ANP treatment; and nuclear-targeted Akt (Akt-n.t.) adenoviral infection with and without pharmacologic inhibitors. *P < 0.005 relative to the control (first bar of each group) was found as indicated. Myocardial sections (C–L) show nuclear accumulation of zyxin in transgenic mice expressing cardiac-specific nuclear-targeted Akt (Akt-n.t. Tg) but not in nontransgenic control samples. Arrows indicate nuclei positive for both zyxin and Akt-n.t. Transgenic Akt-n.t. was visualized using antibody to myc-tag (tag) and desmin to show myofibrillar structure. Arrows indicate nuclei positive for both Akt-n.t. and zyxin. Nuclei were shown to correlate with Akt-n.t. labeling but are not included in overlay to facilitate presentation. Overlay colors correspond to Akt-n.t. (red), zyxin (green), and desmin (blue) with coincidence of red and blue labeling appearing yellow. Scale bars: 20 μm (C–L).
Similar articles
- Cardioprotective stimuli mediate phosphoinositide 3-kinase and phosphoinositide dependent kinase 1 nuclear accumulation in cardiomyocytes.
Rubio M, Avitabile D, Fischer K, Emmanuel G, Gude N, Miyamoto S, Mishra S, Schaefer EM, Brown JH, Sussman MA. Rubio M, et al. J Mol Cell Cardiol. 2009 Jul;47(1):96-103. doi: 10.1016/j.yjmcc.2009.02.022. Epub 2009 Mar 6. J Mol Cell Cardiol. 2009. PMID: 19269295 Free PMC article. - Akt phosphorylation of zyxin mediates its interaction with acinus-S and prevents acinus-triggered chromatin condensation.
Chan CB, Liu X, Tang X, Fu H, Ye K. Chan CB, et al. Cell Death Differ. 2007 Sep;14(9):1688-99. doi: 10.1038/sj.cdd.4402179. Epub 2007 Jun 15. Cell Death Differ. 2007. PMID: 17572661 - Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes.
Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Shiraishi I, et al. Circ Res. 2004 Apr 16;94(7):884-91. doi: 10.1161/01.RES.0000124394.01180.BE. Epub 2004 Feb 26. Circ Res. 2004. PMID: 14988230 - The emerging multiple roles of nuclear Akt.
Martelli AM, Tabellini G, Bressanin D, Ognibene A, Goto K, Cocco L, Evangelisti C. Martelli AM, et al. Biochim Biophys Acta. 2012 Dec;1823(12):2168-78. doi: 10.1016/j.bbamcr.2012.08.017. Epub 2012 Aug 31. Biochim Biophys Acta. 2012. PMID: 22960641 Review. - cGMP signalling in cardiomyocyte microdomains.
Bork NI, Molina CE, Nikolaev VO. Bork NI, et al. Biochem Soc Trans. 2019 Oct 31;47(5):1327-1339. doi: 10.1042/BST20190225. Biochem Soc Trans. 2019. PMID: 31652306 Review.
Cited by
- Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice.
Azibani F, Devaux Y, Coutance G, Schlossarek S, Polidano E, Fazal L, Merval R, Carrier L, Solal AC, Chatziantoniou C, Launay JM, Samuel JL, Delcayre C. Azibani F, et al. PLoS One. 2012;7(5):e38197. doi: 10.1371/journal.pone.0038197. Epub 2012 May 30. PLoS One. 2012. PMID: 22666483 Free PMC article. - Cell density-dependent proteolysis by HtrA1 induces translocation of zyxin to the nucleus and increased cell survival.
Sabino F, Madzharova E, Auf dem Keller U. Sabino F, et al. Cell Death Dis. 2020 Aug 21;11(8):674. doi: 10.1038/s41419-020-02883-2. Cell Death Dis. 2020. PMID: 32826880 Free PMC article. - Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena.
Zinchuk V, Zinchuk O, Okada T. Zinchuk V, et al. Acta Histochem Cytochem. 2007 Aug 30;40(4):101-11. doi: 10.1267/ahc.07002. Acta Histochem Cytochem. 2007. PMID: 17898874 Free PMC article. - Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures.
Hoshijima M. Hoshijima M. Am J Physiol Heart Circ Physiol. 2006 Apr;290(4):H1313-25. doi: 10.1152/ajpheart.00816.2005. Am J Physiol Heart Circ Physiol. 2006. PMID: 16537787 Free PMC article. Review. - Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling.
Burley DS, Ferdinandy P, Baxter GF. Burley DS, et al. Br J Pharmacol. 2007 Nov;152(6):855-69. doi: 10.1038/sj.bjp.0707409. Epub 2007 Aug 13. Br J Pharmacol. 2007. PMID: 17700722 Free PMC article. Review.
References
- Pidgeon GB, et al. Differing metabolism and bioactivity of atrial and brain natriuretic peptides in essential hypertension. Hypertension. 1996;27:906–913. - PubMed
- Tonolo G, et al. Low-dose infusion of atrial natriuretic factor in mild essential hypertension. Circulation. 1989;80:893–902. - PubMed
- Hayashi M, et al. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2001;37:1820–1826. - PubMed
- Lai CP, et al. Beneficial effects of atrial natriuretic peptide on exercise-induced myocardial ischemia in patients with stable effort angina pectoris. Circulation. 1993;87:144–151. - PubMed
- Rosenthal AD, Moran M, Herrmann HC. Coronary hemodynamic effects of atrial natriuretic peptide in humans. J. Am. Coll. Cardiol. 1990;16:1107–1113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS/HL 025037/NS/NINDS NIH HHS/United States
- GM50877/GM/NIGMS NIH HHS/United States
- HL075575/HL/NHLBI NIH HHS/United States
- R01 HL066035/HL/NHLBI NIH HHS/United States
- R01 GM050877/GM/NIGMS NIH HHS/United States
- P01 AG23071/AG/NIA NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- R01 NS025037/NS/NINDS NIH HHS/United States
- HL66035/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- HL67245/HL/NHLBI NIH HHS/United States
- HL58224/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources