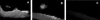The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces - PubMed (original) (raw)
The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces
Elena S Lysenko et al. PLoS Pathog. 2005 Sep.
Abstract
Since mucosal surfaces may be simultaneously colonized by multiple species, the success of an organism may be determined by its ability to compete with co-inhabitants of its niche. To explore the contribution of host factors to polymicrobial competition, a murine model was used to study the initiation of colonization by Haemophilus influenzae and Streptococcus pneumoniae. Both bacterial species, which occupy a similar microenvironment within the nasopharynx, persisted during colonization when given individually. Co-colonization, however, resulted in rapid clearance of S. pneumoniae from the upper respiratory tract, associated with increased recruitment of neutrophils into paranasal spaces. Systemic depletion of either neutrophil-like cells or complement was sufficient to eliminate this competitive effect, indicating that clearance was likely due to enhanced opsonophagocytic killing. The hypothesis that modulation of opsonophagocytic activity was responsible for host-mediated competition was tested using in vitro killing assays with elicited neutrophil-like cells. Components of H. influenzae (but not S. pneumoniae) stimulated complement-dependent phagocytic killing of S. pneumoniae. Thus, the recruitment and activation of neutrophils through selective microbial pattern recognition may underlie the H. influenzae-induced clearance of S. pneumoniae. This study demonstrates how innate immune responses may mediate competitive interactions between species and dictate the composition of the colonizing flora.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
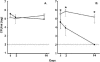
Figure 1. Colonization of BALB/c SCID Mice by H. influenzae Strain _Hi_636 and S. pneumoniae Strain _Sp_1121
The mean density of H. influenzae strain _Hi_636 (A) and S. pneumoniae strain _Sp_1121 (B) in upper respiratory tract lavage ± standard deviation was determined on the day indicated following intranasal inoculation with 1 × 107 CFU of either one (open symbols) or both species (solid symbols). * p < 0.01 compared to co-colonization. Dashed line denotes the lower limit of detection.
Figure 2. Immunofluorescence Showing Co-Localization of H. influenzae and S. pneumoniae in the Murine Nasopharynx
BALB/c SCID mice were co-colonized with _Hi_636 and _Sp_1121, and at 24 h post-inoculation adjacent 5-μm frozen parasagittal tissue sections through the lateral nasal spaces of the same animals were stained with anti-capsular polysaccharide serum specific to type b H. influenzae (A), type 23F S. pneumoniae (B), or secondary antibody control with no primary antibody (C). Magnification, 400×.
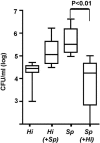
Figure 3. Competition between Species during Co-Colonization of Immunocompetent Mice
The density of H. influenzae strain _Hi_636 (Hi) and S. pneumoniae strain _Sp_1121 (Sp) in upper respiratory tract lavage was determined at 24 h after intranasal inoculation of C3H/HeOuJ mice. Box-and-whiskers plot indicates high and low values, median, and interquartile ranges; n ≥ 10 in each group. Co-inoculated species shown in parentheses. The lower limit of detection for bacteria in lavage culture was 102 CFU/ml.
Figure 4. H&E-Stained Parasagittal Sections Showing the Luminal Space between Two Adjacent Nasal Turbinates
Representative sections are shown for BALB/c SCID mice mock colonized (A) or co-colonized with H. influenzae strain _Hi_636 and S. pneumoniae strain _Sp_1121 (B); C3H/HeOuJ mice mock colonized (C) or co-colonized with _Hi_636 and _Sp_1121 (D). Arrows indicate cells infiltrating into the lumen of nasal spaces in co-colonized mice. Under higher magnification these cells have the morphologic appearance of neutrophils. Magnification, 400× (insert magnification, 1,000×).
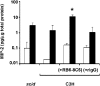
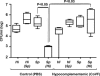
Figure 5. The Effect of Co-Colonization on the Concentration of MIP-2 in Upper Respiratory Tract Lavage Fluid
MIP-2 levels normalized to total protein content were determined in mock colonized (open bars) and dual colonized (solid bars) mice including BALB/c SCID mice, C3H/HeOuJ mice, and C3H/HeOuJ mice treated with RB6-8C5 or rat IgG control as indicated. Values are geometric means ± standard deviation. *p < 0.01 compared to other co-colonized groups of C3H mice. Dual colonized SCID and C3H mice showed significantly higher levels of MIP-2 compared to mock colonized controls (p < 0.03).
Figure 6. The Effect of Depletion of Neutrophil-Like Cells on Competition between Species during Co-Colonization
(A) The density of H. influenzae strain _Hi_636 (Hi) and S. pneumoniae strain _Sp_1121 (Sp) in upper respiratory tract lavage was determined at 24 h after intranasal inoculation in C3H/HeOuJ mice pretreated with RB6-8C5 to deplete neutrophil-like cells or pretreated with rat IgG as a control. Box-and-whiskers plot indicates high and low values, median, and interquartile ranges; n ≥ 5 in each group. Co-inoculated species shown in parentheses. The lower limit of detection for bacteria in lavage culture was 102 CFU/ml. (B) Representative parasagittal sections of the lateral nasal tissues adjacent to the turbinates of co-colonized C3H/HeOuJ mice: (i) untreated control (H&E-stained), (ii) pretreated to deplete neutrophil-like cells (H&E-stained), or (iii) untreated control (stained with mAb Ly-6G to detect neutrophil-like cells). Magnification, 400×.
Figure 7. The Effect of Complement Depletion on Competition between Species during Co-Colonization
The density of H. influenzae strain _Hi_636 (Hi) and S. pneumoniae strain _Sp_1121 (Sp) in upper respiratory tract lavage was determined at 24 h after intranasal inoculation in C3H/HeOuJ mice pretreated with cobra venom factor (CoVF) to deplete complement or PBS control. Box-and-whiskers plot indicates high and low values, median, and interquartile ranges; n ≥ 5 in each group. Co-inoculated species shown in parentheses. The lower limit of detection for bacteria in lavage culture was 102 CFU/ml.
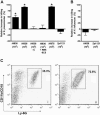
Figure 8. Stimulation of Neutrophil-Like Cells and Opsonophagocytic Killing by Components of H. influenzae
Killing of S. pneumoniae strain _Sp_1121 (A) or H. influenzae strain _Hi_636 (B) by neutrophil-enriched PECs was determined over a 45-min incubation with active complement. The effect on killing by neutrophil-enriched PECs of i.p. administration of heat-inactivated whole bacteria at the dose indicated is shown relative to controls consisting of casein administration alone. No stimulation of killing was observed in controls using inactivated complement (−C) or cells from animals pretreated with mAb RB6-5C8. Values represent three or more independent determinations in duplicate ± standard error of the mean of the percent killing relative to controls using neutrophil-enriched PECs elicited without the addition of heat-inactivated bacteria. *p < 0.001 compared to groups without heat-inactivated H. influenzae and active complement. (C) A representative FACS analysis of neutrophil-enriched PECs showing the effect of i.p. administration of casein alone (i) or with 106 heat-inactivated _Hi_636 (ii) on the proportion of Ly-6G positive cells co-expressing the activation marker CD11b/CD18 (boxed).
Similar articles
- Within-host competition drives selection for the capsule virulence determinant of Streptococcus pneumoniae.
Lysenko ES, Lijek RS, Brown SP, Weiser JN. Lysenko ES, et al. Curr Biol. 2010 Jul 13;20(13):1222-6. doi: 10.1016/j.cub.2010.05.051. Epub 2010 Jun 17. Curr Biol. 2010. PMID: 20619820 Free PMC article. - Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing.
Lysenko ES, Clarke TB, Shchepetov M, Ratner AJ, Roper DI, Dowson CG, Weiser JN. Lysenko ES, et al. PLoS Pathog. 2007 Aug 24;3(8):e118. doi: 10.1371/journal.ppat.0030118. PLoS Pathog. 2007. PMID: 17722978 Free PMC article. - Microbial interactions in the respiratory tract.
Murphy TF, Bakaletz LO, Smeesters PR. Murphy TF, et al. Pediatr Infect Dis J. 2009 Oct;28(10 Suppl):S121-6. doi: 10.1097/INF.0b013e3181b6d7ec. Pediatr Infect Dis J. 2009. PMID: 19918134 Review. - Haemophilus influenzae and Streptococcus pneumoniae: living together in a biofilm.
Tikhomirova A, Kidd SP. Tikhomirova A, et al. Pathog Dis. 2013 Nov;69(2):114-26. doi: 10.1111/2049-632X.12073. Epub 2013 Sep 10. Pathog Dis. 2013. PMID: 23913525 Review.
Cited by
- Alterations of Bacteroides sp., Neisseria sp., Actinomyces sp., and Streptococcus sp. populations in the oropharyngeal microbiome are associated with liver cirrhosis and pneumonia.
Lu H, Qian G, Ren Z, Zhang C, Zhang H, Xu W, Ye P, Yang Y, Li L. Lu H, et al. BMC Infect Dis. 2015 Jun 23;15:239. doi: 10.1186/s12879-015-0977-x. BMC Infect Dis. 2015. PMID: 26099252 Free PMC article. - Role of pore-forming toxins in bacterial infectious diseases.
Los FC, Randis TM, Aroian RV, Ratner AJ. Los FC, et al. Microbiol Mol Biol Rev. 2013 Jun;77(2):173-207. doi: 10.1128/MMBR.00052-12. Microbiol Mol Biol Rev. 2013. PMID: 23699254 Free PMC article. Review. - Staphylococcus epidermidis Protection Against Staphylococcus aureus Colonization in People Living With Human Immunodeficiency Virus in an Inner-City Outpatient Population: A Cross-Sectional Study.
Sullivan SB, Kamath S, McConville TH, Gray BT, Lowy FD, Gordon PG, Uhlemann AC. Sullivan SB, et al. Open Forum Infect Dis. 2016 Dec 20;3(4):ofw234. doi: 10.1093/ofid/ofw234. eCollection 2016 Oct. Open Forum Infect Dis. 2016. PMID: 28018932 Free PMC article. - Deciphering the tête-à-tête between the microbiota and the immune system.
Surana NK, Kasper DL. Surana NK, et al. J Clin Invest. 2014 Oct;124(10):4197-203. doi: 10.1172/JCI72332. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036709 Free PMC article. Review.
References
- Nataro J, Cohen P, Mobley H, Weiser J, editors. Colonization of mucosal surfaces. Washington (DC): ASM Press; 2005. 456. p.
- Ratner A. Competitive and cooperative interactions in the respiratory microflora. In: Nataro J, Cohen P, Mobley H, Weiser J, editors. Colonization of mucosal surfaces. Washington (DC): ASM Press; 2005. pp. 87–96.
- Shakhnovich E, King S, Weiser J. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: A paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect Immun. 2002;70:7161–7164. - PMC - PubMed
Grants and funding
- R01 AI044231/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- R21 AI044231/AI/NIAID NIH HHS/United States
- R37 AI038446/AI/NIAID NIH HHS/United States
- R01 AI038446/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources