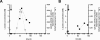Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production - PubMed (original) (raw)
Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production
Corinna Stansen et al. Appl Environ Microbiol. 2005 Oct.
Abstract
Gene expression changes of glutamate-producing Corynebacterium glutamicum were identified in transcriptome comparisons by DNA microarray analysis. During glutamate production induced by a temperature shift, C. glutamicum strain 2262 showed significantly higher mRNA levels of the NCgl2816 and NCgl2817 genes than its non-glutamate-producing derivative 2262NP. Reverse transcription-PCR analysis showed that the two genes together constitute an operon. NCgl2816 putatively codes for a lactate permease, while NCgl2817 was demonstrated to encode quinone-dependent l-lactate dehydrogenase, which was named LldD. C. glutamicum LldD displayed Michaelis-Menten kinetics for the substrate l-lactate with a K(m) of about 0.51 mM. The specific activity of LldD was about 10-fold higher during growth on l-lactate or on an l-lactate-glucose mixture than during growth on glucose, d-lactate, or pyruvate, while the specific activity of quinone-dependent d-lactate dehydrogenase differed little with the carbon source. RNA levels of NCgl2816 and lldD were about 18-fold higher during growth on l-lactate than on pyruvate. Disruption of the NCgl2816-lldD operon resulted in loss of the ability to utilize l-lactate as the sole carbon source. Expression of lldD restored l-lactate utilization, indicating that the function of the permease gene NCgl2816 is dispensable, while LldD is essential, for growth of C. glutamicum on l-lactate.
Figures
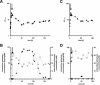
FIG. 1.
Continuous culture of glutamate-producing C. glutamicum 2262 (A and B) and its non-glutamate-producing mutant 2262NP (C and D) at 39°C and a dilution rate of 0.05 h−1. (A and C) Optical densities at 570 nm (▴); (B and D) intracellular (×) and extracellular (⧫) glutamate concentrations. Arrows indicate the time when both the continuous supplementation of medium and the temperature increase from 33 to 39°C were imposed. The pH was maintained at 7.
FIG. 2.
l
-Lactate concentrations in supernatants (○) and specific activities of quinone-dependent
l
-lactate dehydrogenase (▪) in crude extracts of glutamate-producing C. glutamicum 2262 (A) and of its non-glutamate-producing mutant 2262NP (B) during continuous culture. Samples analyzed were taken from the continuous cultures described in the legend to Fig. 1.
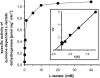
FIG. 3.
Substrate dependence of C. glutamicum WT(pEKEx3-lldD) quinone-dependent
l
-lactate dehydrogenase. Shown are specific activities of quinone-dependent
l
-lactate dehydrogenase in crude extracts from C. glutamicum WT(pEKEx3-lldD) with varying concentrations of the substrate
l
-lactate. (Inset) Double-reciprocal plot of the data.
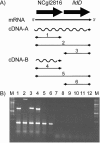
FIG. 4.
Transcriptional organization of the NCgl2816_-lldD_ locus in C. glutamicum analyzed by RT-PCR. (A) Scheme showing the NCgl2816_-lldD_ locus in C. glutamicum and the RT-PCRs used to determine cotranscription of NCgl2816 and lldD. RNA from wild-type C. glutamicum was transcribed into cDNA with two different primers in the two separate reverse transcriptase reactions cDNA-A and cDNA-B. Subsequently, these cDNAs were used as templates for the PCRs labeled 1 to 6. (B) Results from the RT-PCR analyses described above. The lower DNA fragment visible in lanes 1 to 6 represents dnaE, and RT-PCR of dnaE served as a positive control in all reactions. The upper bands correspond to the products of PCRs 1 to 6, diagramed in panel A. Lanes 7 to 12 represent control reactions confirming the absence of DNA in the RNA preparation. The reactions were identical to PCRs 1 to 6 (for which results are shown in lanes 1 to 6, respectively) except that reverse transcriptase was omitted in reactions cDNA-A and cDNA-B.
Similar articles
- Regulation of L-lactate utilization by the FadR-type regulator LldR of Corynebacterium glutamicum.
Georgi T, Engels V, Wendisch VF. Georgi T, et al. J Bacteriol. 2008 Feb;190(3):963-71. doi: 10.1128/JB.01147-07. Epub 2007 Nov 26. J Bacteriol. 2008. PMID: 18039772 Free PMC article. - Quinone-dependent D-lactate dehydrogenase Dld (Cg1027) is essential for growth of Corynebacterium glutamicum on D-lactate.
Kato O, Youn JW, Stansen KC, Matsui D, Oikawa T, Wendisch VF. Kato O, et al. BMC Microbiol. 2010 Dec 15;10:321. doi: 10.1186/1471-2180-10-321. BMC Microbiol. 2010. PMID: 21159175 Free PMC article. - A role of the transcriptional regulator LldR (NCgl2814) in glutamate metabolism under biotin-limited conditions in Corynebacterium glutamicum.
Supkulsutra T, Maeda T, Kumagai K, Wachi M. Supkulsutra T, et al. J Gen Appl Microbiol. 2013;59(3):207-14. doi: 10.2323/jgam.59.207. J Gen Appl Microbiol. 2013. PMID: 23863291 - The global repressor SugR controls expression of genes of glycolysis and of the L-lactate dehydrogenase LdhA in Corynebacterium glutamicum.
Engels V, Lindner SN, Wendisch VF. Engels V, et al. J Bacteriol. 2008 Dec;190(24):8033-44. doi: 10.1128/JB.00705-08. Epub 2008 Oct 10. J Bacteriol. 2008. PMID: 18849435 Free PMC article.
Cited by
- Review of the Proteomics and Metabolic Properties of Corynebacterium glutamicum.
Park J, Lim S. Park J, et al. Microorganisms. 2024 Aug 15;12(8):1681. doi: 10.3390/microorganisms12081681. Microorganisms. 2024. PMID: 39203523 Free PMC article. Review. - Exploring the role of sigma factor gene expression on production by Corynebacterium glutamicum: sigma factor H and FMN as example.
Taniguchi H, Wendisch VF. Taniguchi H, et al. Front Microbiol. 2015 Jul 22;6:740. doi: 10.3389/fmicb.2015.00740. eCollection 2015. Front Microbiol. 2015. PMID: 26257719 Free PMC article. - Fermentative Indole Production via Bacterial Tryptophan Synthase Alpha Subunit and Plant Indole-3-Glycerol Phosphate Lyase Enzymes.
Ferrer L, Mindt M, Suarez-Diez M, Jilg T, Zagorščak M, Lee JH, Gruden K, Wendisch VF, Cankar K. Ferrer L, et al. J Agric Food Chem. 2022 May 11;70(18):5634-5645. doi: 10.1021/acs.jafc.2c01042. Epub 2022 May 2. J Agric Food Chem. 2022. PMID: 35500281 Free PMC article. - Rational Engineering of Non-Ubiquinone Containing Corynebacterium glutamicum for Enhanced Coenzyme Q10 Production.
Burgardt A, Pelosi L, Chehade MH, Wendisch VF, Pierrel F. Burgardt A, et al. Metabolites. 2022 May 11;12(5):428. doi: 10.3390/metabo12050428. Metabolites. 2022. PMID: 35629932 Free PMC article. - Gene expression profiling of Corynebacterium glutamicum during Anaerobic nitrate respiration: induction of the SOS response for cell survival.
Nishimura T, Teramoto H, Inui M, Yukawa H. Nishimura T, et al. J Bacteriol. 2011 Mar;193(6):1327-33. doi: 10.1128/JB.01453-10. Epub 2011 Jan 14. J Bacteriol. 2011. PMID: 21239583 Free PMC article.
References
- Bott, M., and A. Niebisch. 2003. The respiratory chain of Corynebacterium glutamicum. J. Biotechnol. 104:129-153. - PubMed
- Delaunay, S., P. Daran-Lapujade, J. M. Engasser, and J. L. Goergen. 2004. Glutamate as an inhibitor of phosphoenolpyruvate carboxylase activity in Corynebacterium glutamicum. J. Ind. Microbiol. Biotechnol. 31:183-188. - PubMed
- Delaunay, S., P. Gourdon, P. Lapujade, E. Mailly, E. Oriol, J. M. Engasser, N. L. Lindley, and J. L. Goergen. 1999. An improved temperature triggered process for glutamate production with Corynebacterium glutamicum. Enzyme Microb. Biotechnol. 25:762-768.
- Eggeling, L., K. Krumbach, and H. Sahm. 2001. l-Glutamate efflux with Corynebacterium glutamicum: why is penicillin treatment or Tween addition doing the same? J. Mol. Microbiol. Biotechnol. 3:67-68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases