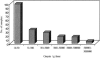Patterns of Cryptosporidium oocyst shedding by eastern grey kangaroos inhabiting an Australian watershed - PubMed (original) (raw)
Patterns of Cryptosporidium oocyst shedding by eastern grey kangaroos inhabiting an Australian watershed
Michelle L Power et al. Appl Environ Microbiol. 2005 Oct.
Abstract
The occurrence of Cryptosporidium oocysts in feces from a population of wild eastern grey kangaroos inhabiting a protected watershed in Sydney, Australia, was investigated. Over a 2-year period, Cryptosporidium oocysts were detected in 239 of the 3,557 (6.7%) eastern grey kangaroo fecal samples tested by using a combined immunomagnetic separation and flow cytometric technique. The prevalence of Cryptosporidium in this host population was estimated to range from 0.32% to 28.5%, with peaks occurring during the autumn months. Oocyst shedding intensity ranged from below 20 oocysts/g feces to 2.0 x 10(6) oocysts/g feces, and shedding did not appear to be associated with diarrhea. Although morphologically similar to the human-infective Cryptosporidium hominis and the Cryptosporidium parvum "bovine" genotype oocysts, the oocysts isolated from kangaroo feces were identified as the Cryptosporidium "marsupial" genotype I or "marsupial" genotype II. Kangaroos are the predominant large mammal inhabiting Australian watersheds and are potentially a significant source of Cryptosporidium contamination of drinking water reservoirs. However, this host population was predominantly shedding the marsupial-derived genotypes, which to date have been identified only in marsupial host species.
Figures
FIG. 1.
Distribution of oocyst numbers shed in 239 positive fecal samples from eastern grey kangaroos. The distribution of Cryptosporidium in this host is highly aggregated (k < 1).
FIG. 2.
Distribution of Cryptosporidium marsupial genotypes for each fecal sample collection period. (No samples were collected in August 2001 due to bushfires preventing access.)
References
- Anderson, R. M. 1993. Epidemiology, p. 75-114. In F. E. G. Cox (ed.), Modern parasitology. Blackwell Science, London, United Kingdom.
- Atwill, E. R., S. M. Camargo, R. Phillips, L. H. Alonso, K. W. Tate, W. A. Jensen, J. Bennet, S. Little, and T. P. Salmon. 2001. Quantitative shedding of two genotypes of Cryptosporidium parvum in California ground squirrels Spermophilus beecheyi. Appl. Environ. Microbiol. 67**:**2840-2843. - PMC - PubMed
- Atwill, E. R., E. Johnson, D. J. Klingborg, G. M. Veserat, G. Markegard, W. A. Jensen, D. W. Pratt, R. E. Delmas, H. A. George, L. C. Forero, R. L. Philips, S. J. Barry, N. K. McDougald, R. R. Gildersleeve, and W. E. Frost. 1999. Age, geographic and temporal distribution of fecal shedding of Cryptosporidium in cow-calf herds. Am. J. Vet. Res. 60**:**420-425. - PubMed
- Barger, I. A. 1985. The statistical distribution of trichostrongylid nematodes in grazing lambs. Int. J. Parasitol. 15**:**645-649. - PubMed
- Bodley-Tickell, A. T., S. E. Kitchen, and A. P. Sturdee. 2002. Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Res. 36**:**1880-1886. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical