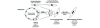Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis - PubMed (original) (raw)
Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis
Vineeth S Rajkumar et al. Arthritis Res Ther. 2005.
Abstract
The mechanisms by which microvascular damage leads to dermal fibrosis in diffuse cutaneous systemic sclerosis (dcSSc) are unclear. We hypothesized that microvascular pericytes constitute a cellular link between microvascular damage and fibrosis by transdifferentiating into myofibroblasts. We used a combination of immunohistochemistry and double immunofluorescence labelling of frozen skin biopsies taken from normal and dcSSc patients to determine whether a phenotypic link between pericytes and myofibroblasts exists in dcSSc. Using alpha-smooth muscle actin, the ED-A splice variant of fibronectin (ED-A FN) and Thy-1 to identify myofibroblasts, we demonstrated the presence of myofibroblasts in fibrotic dcSSc skin. Myofibroblasts were totally absent from control skin, atrophic stage dcSSc skin and non-lesional skin. Using double immunofluorescence labelling, both myofibroblasts and pericytes were shown to express ED-A FN and Thy-1 in dcSSc skin but not in control skin. Proliferating cell nuclear antigen was also expressed by myofibroblasts and pericytes in dcSSc skin while being absent in control skin. These observations suggest that the presence of myofibroblasts may represent a transitional phase during the fibrotic stages of dcSSc and that Thy-1+ve pericytes participate in the fibrogenic development of dcSSc by synthesizing ED-A FN, which may be associated with a proliferation and transition of pericytes and fibroblasts to myofibroblasts, thus linking microvascular damage and fibrosis.
Figures
Figure 1
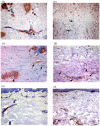
Detection of myofibroblasts in dcSSc skin. Cryosections from (a) normal and (b-f) dcSSc skin were stained with an antibody against α-SMA. In normal skin, α-SMA staining was restricted primarily to microvascular pericytes enveloping capillaries ((a) arrows), sweat glands ((a) black arrowhead) and smooth muscle cells of erector pili muscles ((a) white arrowhead). In dcSSc samples, α-SMA-expressing myofibroblasts were detected in the dermis ((b,c,d) black arrows). Myofibroblasts were predominantly detected in the lower reticular dermis of SSc skin ((c,d) black arrows) while interstitial cells in the papillary dermis did not express α-SMA ((c,d) white arrows). In reticular dermal layers, α-SMA staining was also detected in the perivascular region ((c,d) black arrowheads) while in the papillary dermal layers α-SMA immunostaining was restricted to microvessels ((c) white arrowhead). In (e) non-lesional and (f) late stage dcSSc, the distribution of α-SMA was similar to that seen in normal skin. Original magnification (a,b,e,f) ×10, and (c,d) × 20. α-SMA, alpha smooth muscle actin; dcSSc, diffuse cutaneous systemic sclerosis.
Figure 2
Increased expression of LOX and ED-A FN in dcSSc skin. Cryosections of (a,b) normal skin are compared with (c-f) dcSSc skin. In normal skin, immunostaining for LOX was detected in epidermal cells ((a) arrow). In dcSSc skin, immunostaining for LOX was detected in fibroblast-like cells throughout the dermis ((c,e) arrows) and in cells of the microvascular wall ((e) arrowhead). Little or no expression of ED-A FN was detectable in (b) normal skin, however, ED-A FN immunostaining was markedly increased in dcSSc skin ((d,f) arrows). Immunostaining for ED-A FN was also detected in cells of the microvascular wall ((f) arrowhead). Original magnification (a-d) × 10 and (e,f) × 20. dcSSc, diffuse cutaneous systemic sclerosis; ED-A FN, ED-A splice variant of fibronectin; LOX, lysyl oxidase.
Figure 3
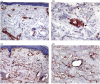
Expression of ED-A correlates specifically with myofibroblasts in dcSSc skin. (a,c) Serial cryosections were stained with antibodies against ED-A FN and (b,d) α-SMA. Both ED-A FN ((a,c) arrows) and α-SMA+ve myofibroblasts ((b,d) arrows) were predominant in the lower reticular dermis of dcSSc skin. Note the absence of ED-A FN ((a) white arrow) and α-SMA+ve myofibroblasts ((b) white arrow) in the papillary dermis. In addition, immunostaining for ED-A FN was also detected in the wall of microvessels ((c) inset, arrowheads) correspondingly containing α-SMA-expressing pericytes ((d) inset, arrowheads). Original magnification (a,b) × 10, (c,d) × 20, inset (c,d) × 40. α-SMA, alpha smooth muscle actin; dcSSc, diffuse cutaneous systemic sclerosis; ED-A FN, ED-A splice variant of fibronectin.
Figure 4
Expression of Thy-1 is increased in dcSSc skin. Cryosections from (a,b) normal and (c,d) dcSSc were stained for Thy-1 expression. In normal skin, immunostaining for Thy-1 was predominantly located within the microvascular wall and immediate perivascular region ((a,b) arrows). Thy-1 staining of interstitial fibroblasts was also detected ((b) arrowhead). In dcSSc skin, immunostaining of fibroblastic cells was considerably more pronounced throughout the interstitial dermis ((c) arrows) while perivascular immunostaining in dcSSc skin ((d) arrow) was less pronounced than that observed in normal skin ((b) arrow). dcSSc, diffuse cutaneous systemic sclerosis.
Figure 5
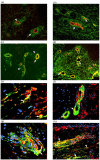
Double immunofluorescence labelling of normal and dcSSc skin biopsies. Cryosections from (a,c) normal and (b,d) dcSSc were double stained for endothelial cells using (a,b) PAL-E antibody and Thy-1 and (c,d) α-SMA and Thy-1. Thy-1 is labelled with FITC while PAL-E and α-SMA are labelled with Texas Red. In both (a) normal and (b) dcSSc, immunofluorescence for Thy-1 ((a,b) arrow, green colour) and PAL-E ((a,b) arrowhead, red colour) was consistently exclusive and showed no colocalization. In both (c) normal and (d) dcSSc, strong colocalization between Thy-1 and α-SMA was evident ((c,d) arrows, yellow colour). In normal skin, Thy-1 immunofluorescence that did not colocalize with α-SMA was observed immediately adjacent to microvessels ((c) arrowheads, green colour). Cryosections from dcSSc were double stained for (e,f,g) ED-A FN and α-SMA and (h) ED-A FN and Thy-1. ED-A FN is labelled with Texas Red while α-SMA and Thy-1 are labelled with FITC. Cell nuclei are counterstained blue with DAPI. Colocalization between α-SMA and ED-A FN was detected in dermal fibroblastic cells ((e) arrows, yellow colour) as well as in the microvascular wall ((f,g) arrows, yellow colour). Colocalization was also observed between ED-A FN and Thy-1 in both the microvascular wall ((h) arrow, yellow colour) and in dermal fibroblastic cells ((h) arrowheads, yellow colour). Original magnification (a-d,h) × 10, (e,f) × 20, (g) × 40. α-SMA, alpha smooth muscle actin; DAPI, 4,6-diamidino-2-phenylindole; dcSSc, diffuse cutaneous systemic sclerosis; ED-A FN, ED-A splice variant of fibronectin; FITC, fluorescein isothiocyanate.
Figure 6
Distribution of proliferating cells in normal and dcSSc skin. Cryosections from (a) normal and (b,c) dcSSc were stained with an anti-PCNA antibody. In normal skin, PCNA immunostaining was restricted to cells within the epidermis and sweat glands ((a,b) arrows). In two out of ten dcSSc samples, PCNA was detected in fibroblastic cells ((b) arrows) and in microvessels ((c) arrows). Double immunofluorescence labelling of dcSSc skin: cryosections were double stained with a combination of antibodies against (d,e) PCNA and α-SMA and (f) PCNA and PAL-E. PCNA is labelled with Texas Red while α-SMA and PAL-E are labelled with FITC. Colocalization was detected with PCNA and α-SMA antibodies within the microvasculature ((d,e) arrows, yellow colour). When used in combination with PAL-E, PCNA-labelled cells ((f) arrows) were predominantly located adjacent and abluminal to endothelial cells ((f) arrowheads). Original magnification × 20. α-SMA, alpha smooth muscle actin; dcSSc, diffuse cutaneous systemic sclerosis; PCNA, proliferating cell nuclear antigen; FITC, fluorescein isothiocyanate.
Figure 7
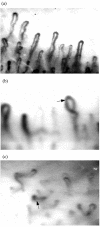
Nailfold capillaroscopy of (a) normal and (b,c) dcSSc patients. In the active pattern of capillary damage, frequent giant capillaries are present ((b) arrow) accompanied by moderate capillary loss and disorganisation of capillary architecture. Late disease pattern was characterized by severe capillary disorganisation with loss of capillaries ((c) arrow). Magnification ×150. dcSSc, diffuse cutaneous systemic sclerosis.
Figure 8
Convergence of microvascular pericytes and resident fibroblasts to a myofibroblast lineage in SSc. Two pathways potentially contribute to the fibrogenic response in dcSSc. Microvascular pericytes (Thy-1+ve /α-SMA+ve ) become activated as a result of microvascular damage and produce the ED-A splice variant of fibronectin, a protein known to induce the myofibroblast phenotype. The microvascular derived ED-A FN in concert with the actions TGF-β may also act upon resident perivascular fibroblasts (Thy-1+ve /α-SMA-ve ) stimulating their differentiation to myofibroblasts. Proliferation of both pericytes and fibroblasts may help to create a pool of potential myofibroblasts. α-SMA, alpha smooth muscle actin; dcSSc, diffuse cutaneous systemic sclerosis; ED-A FN, ED-A splice variant of fibronectin; TGF-β, transforming growth factor-beta.
Similar articles
- Perivascular Cells in Diffuse Cutaneous Systemic Sclerosis Overexpress Activated ADAM12 and Are Involved in Myofibroblast Transdifferentiation and Development of Fibrosis.
Cipriani P, Di Benedetto P, Ruscitti P, Liakouli V, Berardicurti O, Carubbi F, Ciccia F, Guggino G, Zazzeroni F, Alesse E, Triolo G, Giacomelli R. Cipriani P, et al. J Rheumatol. 2016 Jul;43(7):1340-9. doi: 10.3899/jrheum.150996. Epub 2016 Jun 1. J Rheumatol. 2016. PMID: 27252423 - Chemokine receptor CCR2 expression by systemic sclerosis fibroblasts: evidence for autocrine regulation of myofibroblast differentiation.
Carulli MT, Ong VH, Ponticos M, Shiwen X, Abraham DJ, Black CM, Denton CP. Carulli MT, et al. Arthritis Rheum. 2005 Dec;52(12):3772-82. doi: 10.1002/art.21396. Arthritis Rheum. 2005. PMID: 16320328 - Decreased cathepsin V expression due to Fli1 deficiency contributes to the development of dermal fibrosis and proliferative vasculopathy in systemic sclerosis.
Noda S, Asano Y, Takahashi T, Akamata K, Aozasa N, Taniguchi T, Ichimura Y, Toyama T, Sumida H, Kuwano Y, Yanaba K, Tada Y, Sugaya M, Kadono T, Sato S. Noda S, et al. Rheumatology (Oxford). 2013 May;52(5):790-9. doi: 10.1093/rheumatology/kes379. Epub 2013 Jan 3. Rheumatology (Oxford). 2013. PMID: 23287360 - The Vessels Contribute to Fibrosis in Systemic Sclerosis.
Di Benedetto P, Ruscitti P, Liakouli V, Cipriani P, Giacomelli R. Di Benedetto P, et al. Isr Med Assoc J. 2019 Jul;21(7):471-474. Isr Med Assoc J. 2019. PMID: 31507123 Review. - Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease.
Kramann R, DiRocco DP, Humphreys BD. Kramann R, et al. J Pathol. 2013 Nov;231(3):273-89. doi: 10.1002/path.4253. J Pathol. 2013. PMID: 24006178 Review.
Cited by
- Thy-1 plays a pathogenic role and is a potential biomarker for skin fibrosis in scleroderma.
Marangoni RG, Datta P, Paine A, Duemmel S, Nuzzo M, Sherwood L, Varga J, Ritchlin C, Korman BD. Marangoni RG, et al. JCI Insight. 2022 Oct 10;7(19):e149426. doi: 10.1172/jci.insight.149426. JCI Insight. 2022. PMID: 36066980 Free PMC article. - Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells.
Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB. Popova AP, et al. Am J Physiol Lung Cell Mol Physiol. 2010 Jun;298(6):L735-43. doi: 10.1152/ajplung.00347.2009. Epub 2010 Feb 26. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20190033 Free PMC article. - New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma.
Abraham DJ, Eckes B, Rajkumar V, Krieg T. Abraham DJ, et al. Curr Rheumatol Rep. 2007 May;9(2):136-43. doi: 10.1007/s11926-007-0008-z. Curr Rheumatol Rep. 2007. PMID: 17502044 Review. - The role of adipokines in systemic sclerosis: a missing link?
Żółkiewicz J, Stochmal A, Rudnicka L. Żółkiewicz J, et al. Arch Dermatol Res. 2019 May;311(4):251-263. doi: 10.1007/s00403-019-01893-1. Epub 2019 Feb 26. Arch Dermatol Res. 2019. PMID: 30806766 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous