Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus - PubMed (original) (raw)
Comparative Study
Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus
Xiao-Bo Liu et al. J Neurosci. 2005.
Abstract
Plexin signaling is required for stereotyped pruning of long axon collaterals in the vertebrate CNS; however, a cellular basis for plexins on stereotyped pruning has not been determined. Using quantitative electron microscopy and immunocytochemistry, we found that infrapyramidal mossy fiber axon collaterals form transient synaptic complexes with basal dendrites of CA3 pyramidal cells in the early postnatal mouse hippocampus. At later postnatal ages, these synaptic complexes stop maturing and are removed before stereotyped pruning by a mechanism that does not involve axon degeneration and glial cell engulfment. In knock-out mice that lack plexin-A3 signaling, the synaptic complexes continue to mature, and, as a result, the collaterals are not pruned. Thus, our results suggest that intact plexin-A3 signaling contributes to synaptic complex elimination, which is associated with stereotyped axon pruning.
Figures
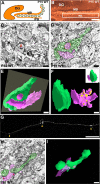
Figure 1.
Synaptic complex formation in the IPB of WT mice before IPB pruning. A, B, Diagram and cross section of the dorsal hippocampus from a WT mouse at P15 that was stained for an antibody for CB. The white square indicates a region of the IPB that was analyzed further with electron microscopy in C-F. DG, Dentate gyrus. C, Electron micrograph of a CB-immunolabeled bouton in the IPB of a WT mouse at P15. The mossy terminal-like bouton (t) forms asymmetrical synapses with a spine (sp) and adendritic shaft (d). An asymmetric synapse on a spine (arrowhead) is shown in higher power in the inset. D, Electron micrograph of a larger P15 WT mossy fiber bouton (green) taken before pruning of the IPB. The bouton establishes several asymmetric synapses (yellow) with a dendritic shaft and its spines (pink). The micrograph was taken from one of 23 thin sections (70 nm per section) from the 3D serial EM reconstruction shown in E (gray square). E, 3D serial reconstruction of the same P15 WT mossy fiber bouton in association with a CA3 dendrite and multiple spines branching off of the dendritic shaft. F, The mossy fiber bouton in E has been rotated 90° and is separated from the postsynaptic elements to display the complex branching pattern of the dendritic spines that establish synaptic contacts (yellow patches) in the central portions of the bouton. G, A laser-scanning confocal microscopic image showing a distal segment of an IPB mossy fiber axon taken from a P15 brain slice that was labeled with DiI. The arrowheads point to en passant giant mossy fiber terminals along the labeled axon. H, Electron micrograph of a CB-immunolabeled longitudinally cut mossy fiber axon from the IPB at P15. The axon (green) contains an en passant bouton that is filled with vesicles and forms asymmetrical synapses (arrowheads) with a dendritic spine and shaft (pink). I, Serial 3D reconstruction of the same axon segment (green) in H. Multiple synapses (yellow patches) are present and associated with a dendritic shaft and spine (pink). Scale bars: B, 150 μm; C, H, I, 0.2 μm, and for inset in C, same scale bar represents 0.1 μm; D-F, 0.5 μm; G, 50 μm.
Figure 2.
Synaptic complex elimination in the IPB of WT mice during IPB pruning. A, B, D, E, Diagrams and cross sections of the dorsal hippocampus from a WT mouse at P25 and P35 that were stained for an antibody for CB. The white squares indicate regions of the IPB that were analyzed further with electron microscopy for P25 (C) and P35 (F). C, P25 IPB, a typical CB-immunolabeled bouton (t) is much smaller compared with boutons at P15 and retains only a single asymmetrical contact with a spine (sp). F, P35 IPB, a smaller CB-immunolabeled bouton (t) retains only a single synapse with a spine (sp). G, Electron micrograph of a small bouton (green) in contact (yellow) with a dendritic spine (pink) taken during pruning of the IPB from a P25 WT mouse (1 of 12 thin sections; 70 nm per section). H, 3D serial reconstruction of the same mossy fiber bouton in G. I, An electron micrograph (1 of 6 thin sections, 70 nm per section) showing a longitudinally cut, CB-immunolabeled axon (green) and one of its en passant boutons taken from a P25 WT mouse during IPB pruning. Microtubules and neurofilaments are still abundant in the axon, but the bouton has regressed in size and is seen containing diffusely distributed vesicles and retaining only one asymmetric synaptic contact (arrowhead) on a dendritic spine that buds off of a small dendritic shaft (pink). J, Serial 3D reconstruction of the same axon in I. The axon (green) is shown with its bouton synapsing (yellow) onto a dendritic spine (pink). Note that the axon caliber is narrower compared with axons at P15 (see Fig. 1 H, I). The bouton is much smaller than at P15 and is shown here retaining only one asymmetric synaptic contact with a dendritic spine. Scale bars: B, E, 150 μm; C, F, I, J, 0.2 μm; G, H, 0.5 μm. DG, Dentate gyrus.
Figure 3.
Loss of PLXA3 signaling results in continued maturation of synaptic complexes in the IPB of PLXA3 and NPN-2 knock-out mice. A-C, Diagram demonstrating the pruning defect observed for mossy fibers in the IPB of PLXA3_-/_- and NPN-2_-/_- mice at P15 (A), P25 (B), and P35 (C). D-F, Immunoelectron micrographs show the ultrastructural features of CB-immunolabeled mossy terminal-like boutons(t) in the IPB at different postnatal ages of PLXA3_-/_- animals. The arrowheads point to asymmetric contacts on spines shown in higher power in the insets. Scale bars: 0.2 μm; insets, same scale bar represents 0.1 μm. G-I, Immunoelectron micrographs show similar ultrastructural features of CB-immunolabeled, mossy terminal-like boutons (t) in the IPB at different postnatal ages of NPN-2_-/_- animals; note that some boutons form multiple asymmetrical contacts with spines (sp) and dendrites (d). The arrowheads point to asymmetric contacts on spines shown in higher power in the insets. Scale bars: 0.2 μm; insets, same scale bar represents 0.1 μm. DG, Dentate gyrus.
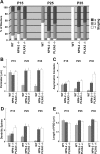
Figure 4.
Quantification of mossy fiber synaptic complexes from WT, NPN-2_-/_-, and PLXA3_-/_- mice. A, Bar graph showing the percentage distribution of mossy fiber synaptic complexes for each phenotype (WT, NPN-2_-/_-, and PLXA3_-/_-) in the IPB at three different postnatal ages. Stage 1 synaptic complexes (white) are classified as containing relatively small and immature mossy fiber boutons with one or no synaptic contacts. Stage 2 synaptic complexes (gray) are classified as intermediate in complexity and contain mossy fiber boutons that establish more synaptic contacts on dendritic shafts than with spines. Stage 3 synaptic complexes (black) contain the most mature and complex mossy fiber boutons that often establish a majority of their synapses on dendritic spines. Staging parameters were modified from a previous study (Amaral and Dent, 1981)(see Materials and Methods). Significant differences between means determined for each grouping were observed for P15 WT versus P25 WT, P15 WT versus P35 WT, and P25-P35 WT versus age-matched P25-P35 NPN-2_-/_-, and PLXA3_-/_- samples (p < 0.05; ANOVA, Newman-Keuls test). **_B_**, Bar graph showing the average perimeter (mean ± SE) of a mossy fiber bouton for each phenotype (WT, _NPN-2_-_/_-, and _PLXA3_-_/_-) at three different postnatal ages. Significant differences between means were observed only between P15 WT versus P25 WT, P15 WT versus P35 WT, and P25-P35 WT versus age-matched P25-P35 _NPN-2_-_/_- and _PLXA3_-_/_- samples (_p_ < 0.05). **_C_**, Bar graph showing the average number (mean ± SE) of asymmetric contacts per bouton for each phenotype (WT, _NPN-2_-_/_-, and _PLXA3_-_/_-) at three different postnatal ages. Significant differences between means were observed only between P15 WT versus P35 WT and P25-P35 WT versus age-matched _NPN-2_-_/_- and _PLXA3_-_/_- samples (_p_ < 0.05). **_D_**, Bar graph showing the average number (mean ± SE) of dendritic spines associated with each mossy fiber bouton for each phenotype (WT, _NPN-2_-_/_-, and _PLXA3_-_/_-) at three different postnatal ages. Significant differences between means were observed only between P25-P35 WT versus age-matched P25-P35 _NPN-2_-_/_- and _PLXA3_-_/_- samples (_p_ < 0.05). **_E_**, Bar graph showing the average PSD length (mean ± SE) for mossy fiber boutons of each phenotype (WT, _NPN-2_-_/_-, and _PLXA3_-_/_-) at three different ages that formed synapses. No significant differences between means were observed in all comparisons of means for each sample (_p_ > 0.10). A-D, A minimum of 15 synaptic complexes were analyzed for each phenotype and age except for P35 WT (n = 10 boutons) in which most synaptic complexes were already removed from the IPB. E, A minimum of 30 PSDs were analyzed for each phenotype and age except for P25 WT (n = 15 PSDs) and P35 WT (n = 4 PSDs).
Figure 5.
Characterization of mossy fiber synaptic complexes of the IPB with synaptic markers. Coronal sections of the dorsal hippocampus were immunostained with VGLUT1 (A, C, E) and VGLUT2 (B, D, F). A, B, P15 WT hippocampi show positive labeling of the MB and IPB for both VGLUT1 and VGLUT2. C, D, P45 WT hippocampi show only positive labeling of the MB for VGLUT1. E, F, P45 NPN-2_-/_- hippocampi show only positive labeling of the IPB for VGLUT1. DG, Dentate gyrus. Scale bar: A-F, 150 μm. G, P15 WT, an immunoelectron micrograph shows VGLUT2 immunoreactivity in association with synaptic vesicles in a mossy terminal-like bouton (t). The bouton forms multiple synaptic contacts with spines (sp) and a dendritic shaft (d) in the IPB. H, P15 WT, an immunoelectron micrograph shows NMDAR1 immunoreactivity localized adjacent to postsynaptic densities (arrowhead) in a dendritic shaft (d), which are in contact with a mossy terminal-like bouton (t) in the IPB. I, P45 NPN-2_-/_-, a mossy terminal-like bouton (t) filled with dense VGLUT1-immunolabeled vesicles makes multiple synaptic contacts with spines (sp) and dendrites (d). J, P45 NPN-2_-/_-, a large bouton (t) makes several synaptic contacts with NMDAR1-immunolabeled dendrites (d) and spines (sp); note that PSDs are associated specifically with NMDAR1 immunoreactivity (arrowheads). Scale bars: G-J, 0.2 μm.
Figure 6.
Synaptic complex elimination occurs before the pruning of mossy fibers in the IPB. A, P15 WT before the pruning of IPB mossy fibers. Coronal sections of dorsal hippocampi were immunostained for CB (left) for the entire mossy fiber projection and Timm's stained (right) for the mossy fiber boutons in the adjacent section. The level of staining of mossy fibers and boutons of the IPB is approximately equal for CB and Timm's staining, respectively. B, P20 WT, an intermediate stage of pruning in which some CB immunolabeling of mossy fibers persists (left), whereas the level of Timm's staining for mossy fiber boutons disappears (right). C, P25 WT, a more completed stage of pruning shows that the level of CB and Timm's staining in the IPB is approximately equal, because the mossy fibers of the IPB have pruned back most of their transient projections to join the MB projections. D, P25 PLXA3 knock-out, a case in which pruning of the IPB does not occur and the levels of CB and Timm's staining are approximately equal. The white arrowheads indicate the level of Timm's or CB labeling in the IPB. Scale bar: A-D, 200 μm. E, A schematic diagram illustrates a model for the cellular mechanism of pruning in the mouse hippocampus. The diagram has been simplified to focus on the synapses associated with en passant mossy fiber boutons that are organized periodically along the axon. In WT and KO (PLXA3_-/_- and NPN-2_-/_-) mice, before pruning occurs, mossy fibers of the IPB (green) form immature synaptic complexes (insets, in left panels of top and bottom rows); boutons (green) are filled with vesicles (black dots) that cluster adjacent to synapses (yellow patches). During the process of pruning, intact PLXA3 signaling for Sema3F causes the gradual elimination of the synaptic complex (top row, middle panel). The elimination of synaptic complexes leads to stereotyped pruning of the IPB (top row, right panel), whereas loss of PLXA3 signaling results in continued maturation of the synaptic complex (bottom row, middle and right panels) and a defect in IPB stereotyped pruning.
Similar articles
- Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex.
Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Low LK, et al. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8136-41. doi: 10.1073/pnas.0803849105. Epub 2008 Jun 3. Proc Natl Acad Sci U S A. 2008. PMID: 18523013 Free PMC article. - Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family.
Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Bagri A, et al. Cell. 2003 May 2;113(3):285-99. doi: 10.1016/s0092-8674(03)00267-8. Cell. 2003. PMID: 12732138 - Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance.
Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H. Suto F, et al. J Neurosci. 2005 Apr 6;25(14):3628-37. doi: 10.1523/JNEUROSCI.4480-04.2005. J Neurosci. 2005. PMID: 15814794 Free PMC article. - Axon pruning in the developing vertebrate hippocampus.
Faulkner RL, Low LK, Cheng HJ. Faulkner RL, et al. Dev Neurosci. 2007;29(1-2):6-13. doi: 10.1159/000096207. Dev Neurosci. 2007. PMID: 17148945 Review. - Axon pruning and synaptic development: how are they per-plexin?
Waimey KE, Cheng HJ. Waimey KE, et al. Neuroscientist. 2006 Oct;12(5):398-409. doi: 10.1177/1073858406292631. Neuroscientist. 2006. PMID: 16957002 Review.
Cited by
- The RacGAP β2-Chimaerin selectively mediates axonal pruning in the hippocampus.
Riccomagno MM, Hurtado A, Wang H, Macopson JG, Griner EM, Betz A, Brose N, Kazanietz MG, Kolodkin AL. Riccomagno MM, et al. Cell. 2012 Jun 22;149(7):1594-606. doi: 10.1016/j.cell.2012.05.018. Cell. 2012. PMID: 22726444 Free PMC article. - Hippocampal gamma rhythms during Y-maze navigation in the juvenile rat.
McHail DG, Dumas TC. McHail DG, et al. Hippocampus. 2020 May;30(5):505-525. doi: 10.1002/hipo.23168. Epub 2019 Oct 18. Hippocampus. 2020. PMID: 31626396 Free PMC article. - Genetics and cell biology of building specific synaptic connectivity.
Shen K, Scheiffele P. Shen K, et al. Annu Rev Neurosci. 2010;33:473-507. doi: 10.1146/annurev.neuro.051508.135302. Annu Rev Neurosci. 2010. PMID: 20367446 Free PMC article. Review. - Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex.
Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Low LK, et al. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8136-41. doi: 10.1073/pnas.0803849105. Epub 2008 Jun 3. Proc Natl Acad Sci U S A. 2008. PMID: 18523013 Free PMC article. - Semaphorin 5B mediates synapse elimination in hippocampal neurons.
O'Connor TP, Cockburn K, Wang W, Tapia L, Currie E, Bamji SX. O'Connor TP, et al. Neural Dev. 2009 May 23;4:18. doi: 10.1186/1749-8104-4-18. Neural Dev. 2009. PMID: 19463192 Free PMC article.
References
- Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195: 51-86. - PubMed
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M (2003) Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell 113: 285-299. - PubMed
- Bernstein M, Lichtman JW (1999) Axonal atrophy: the retraction reaction. Curr Opin Neurobiol 9: 364-370. - PubMed
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW (2004) Axon branch removal at developing synapses by axosome shedding. Neuron 44: 651-661. - PubMed
- Bixby JL, Spitzer NC (1981) Ultrastructural observations on synapse elimination in neonatal rabbit skeletal muscle. J Neurocytol 10: 81-100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous