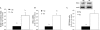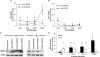Thioredoxin reductase regulates the induction of haem oxygenase-1 expression in aortic endothelial cells - PubMed (original) (raw)
Thioredoxin reductase regulates the induction of haem oxygenase-1 expression in aortic endothelial cells
Wendy L Trigona et al. Biochem J. 2006.
Abstract
Certain selenoproteins such as GPX-1 (glutathione peroxidase-1) and TrxR1 (thioredoxin reductase-1) possess important antioxidant defence functions in vascular endothelial cells. Reduced selenoprotein activity during dietary selenium (Se) deficiency can result in a compensatory increase of other non-Se-dependent antioxidants, such as HO-1 (haem oxygenase-1) that may help to counteract the damaging effects of oxidant stress. However, the role of individual selenoproteins in regulating vascular-derived protective gene responses such as HO-1 is less understood. Using an oxidant stress model based on Se deficiency in BAECs (bovine aortic endothelial cells), we sought to determine whether TrxR1 activity may contribute to the differential regulation of HO-1 expression as a function of altered redox environment. Se-sufficient BAECs up-regulated HO-1 expression following stimulation with the pro-oxidant, 15-HPETE (15-hydroperoxyeicosatetraenoic acid), and levels of this antioxidant inversely correlated with EC apoptosis. While Se-deficient BAECs exhibited higher basal levels of HO-1, it was not up-regulated upon 15-HPETE treatment, which resulted in significantly higher levels of pro-apoptotic markers. Subsequent results showed that HO-1 induction depended on the activity of TrxR1, as proved with chemical inhibitor studies and direct inhibition with TrxR1 siRNA. Finally, restoring intracellular levels of the reduced substrate Trx (thioredoxin) in Sedeficient BAECs was sufficient to increase HO-1 activation following 15-HPETE stimulation. These data provide evidence for the involvement of the Trx/TrxR system, in the regulation of HO-1 expression in BAECs during pro-oxidant challenge.
Figures
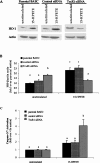
Figure 1. Se deficiency increases expression of HO-1 mRNA and protein
ECs were cultured in +Se or −Se medium for two to three passages. (A) Cells were transiently transfected with HO-1 4.0 kb Luc plasmid and 48 h later were analysed for luciferase activity. Luciferase activity is expressed as fold increase of firefly/Renilla over +Se unstimulated BAECs (unstim). Results are means±S.E.M. for three separate experiments. (B) RNA was isolated and QC RT–PCR was performed. PCR products were separated on a 2% agarose gel, and densitometry was performed in order to quantify the amount of HO-1 mRNA. Results are expressed as fold increase of HO-1 mRNA over +Se unstimulated BAECs (unstim) and are means±S.E.M. for five separate experiments. (C) Whole-cell lysates were harvested, equal amounts of protein were resolved by SDS/12% PAGE, and a Western blot was performed. Results are expressed as fold increase of HO-1 protein/actin ratio over +Se unstimulated BAECs (unstim) and are means±S.E.M. for three separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined using a paired Student's t test.
Figure 2. Differential levels of HO-1 expression in +Se and −Se BAECs following 15-HPETE stimulation
Following stimulation of ECs with dose titration of (A) 15-HPETE or (B) 15-HETE for 2 h, total RNA was isolated, and QC RT–PCR for HO-1 mRNA was then performed. PCR products were separated on a 2% agarose gel, and densitometry of HO-1 mRNA was measured. Results are expressed as fold increase of HO-1 mRNA over +Se unstimulated BAECs (unstim) and are means±S.E.M. for three separate experiments. (C) Following stimulation of ECs with dose titration of 15-HPETE for 2 h, whole-cell lysates were harvested, and equal amounts of protein were resolved by SDS/12% PAGE for Western blot analysis. (D) Densitometry results from three separate experiments in (C) were averaged and means±S.E.M. for three separate experiments are shown. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
Figure 3. HO-1 activity regulates apoptosis during Se deficiency
+Se and −Se BAECs were pre-treated with the indicated doses of the HO-1 inhibitor, SnPPIX, for 15 h, followed by treatment with 30 μM 15-HETE or 15-HPETE for 6 h. (A) Caspase 3/7 activation was measured, and results are expressed as fold increase in caspase-3/7 activation over +Se unstimulated BAECs (unstim) as means±S.E.M. for four separate experiments. (B) For Hoechst 33342 staining, approx. 200 ECs per well, in triplicate per treatment, were manually scored for apoptosis based on the presence or absence of nucleic acid condensation and results are reported as the percentage of BAECs with condensed chromatin. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
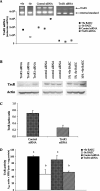
Figure 4. TrxR1 siRNA decreases TrxR mRNA, protein and activity
BAECs were transfected with siRNA for TrxR1 or control sequence and were grown to confluence under antibiotic selection for stable expression of siRNA construct. (A) Total RNA was isolated, and QC RT–PCR for TrxR1 mRNA was then performed. PCR products were separated on a 2% agarose gel, and densitometry of TrxR1 mRNA and internal standard was measured. (B) Whole-cell lysates were harvested, equal amounts of protein were resolved by SDS/10% PAGE, and a Western blot was performed. A representative blot is shown. (C) Results in (B) are expressed as fold increase TrxR protein over actin and are means±S.E.M. for three separate experiments. (D) Whole-cell lysates were harvested and tested for TrxR1 activity using the insulin-based method. Results are expressed in _A_412 units×1000/min per mg of protein, and are means±S.E.M. for three separate clones. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
Figure 5. TrxR1 siRNA inhibits HO-1 induction in BAECs following 15-HPETE stimulation
BAECs were transfected with siRNA for TrxR1 or control sequence and were grown to confluence under antibiotic selection for expression of siRNA construct. (A) Whole-cell lysates from control and TrxR1 siRNA-transfected cells were harvested, and equal amounts of protein were resolved by SDS/12% PAGE for measurement of HO-1 protein levels. (B) Densitometry results from three separate experiments in (A) were averaged, and results are means±S.E.M. for three separate experiments are shown. (C) Caspase 3/7 activation was measured in TrxR siRNA and control siRNA BAECs following 2 h of 15-HPETE stimulation, and results are expressed as fold increase in caspase-3/7 activation over unstimulated BAECs (unstim). Results are means±S.E.M. for four separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
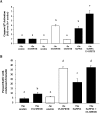
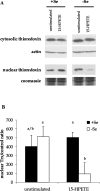
Figure 6. Trx localization following 15-HPETE stimulation
+Se and −Se BAECs were stimulated with 30 μM 15-HPETE or 15-HPETE for 2 h. (A) Cytosolic and nuclear extracts were separated, and equal amounts of protein were resolved by SDS/15% PAGE. (B) Densitometry results from three separate experiments performed with nuclear extracts in (A) were averaged, and results are means±S.E.M. for three separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
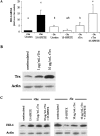
Figure 7. Recombinant Trx induces levels of HO-1 in −Se BAECs following 15-HPETE stimulation
(A) +Se and −Se BAECs were pre-treated with the indicated doses of rTrx for 3 h, followed by treatment with 30 μM 15-HPETE for 2 h. RNA was isolated, and QC RT–PCR was performed. PCR products were separated on a 2% agarose gel, and densitometry was performed in order to quantify the amount of HO-1 mRNA. Results are expressed as fold increase HO-1 mRNA over +Se unstimulated (unstim), and results are means±S.E.M. for three separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA. (B) BAECs were stimulated with 1 or 10 μg/ml rTrx for 3 h, whole-cell lysates were harvested, and equal amounts of protein were resolved by SDS/15% PAGE. A Western blot was performed to confirm increased intracellular Trx protein following rTrx treatment. (C) +Se and −Se BAECs were pre-treated with 10 μg/ml rTrx for 3 h followed by treatment with 30 μM 15-HPETE for 2 h. Whole-cell lysates were harvested, equal amounts of protein were resolved by SDS/12.5% PAGE and a Western blot was performed for HO-1 protein levels. A representative blot of three is shown.
Similar articles
- Loss of activity of the selenoenzyme thioredoxin reductase causes induction of hepatic heme oxygenase-1.
Mostert V, Hill KE, Burk RF. Mostert V, et al. FEBS Lett. 2003 Apr 24;541(1-3):85-8. doi: 10.1016/s0014-5793(03)00309-0. FEBS Lett. 2003. PMID: 12706824 - Effects of selenium deficiency on expression of selenoproteins in bovine arterial endothelial cells.
Hara S, Shoji Y, Sakurai A, Yuasa K, Himeno S, Imura N. Hara S, et al. Biol Pharm Bull. 2001 Jul;24(7):754-9. doi: 10.1248/bpb.24.754. Biol Pharm Bull. 2001. PMID: 11456113 - Selenium-induced antioxidant protection recruits modulation of thioredoxin reductase during excitotoxic/pro-oxidant events in the rat striatum.
Maldonado PD, Pérez-De La Cruz V, Torres-Ramos M, Silva-Islas C, Lecona-Vargas R, Lugo-Huitrón R, Blanco-Ayala T, Ugalde-Muñiz P, Vázquez-Cervantes GI, Fortoul TI, Ali SF, Santamaría A. Maldonado PD, et al. Neurochem Int. 2012 Jul;61(2):195-206. doi: 10.1016/j.neuint.2012.05.004. Epub 2012 May 9. Neurochem Int. 2012. PMID: 22579569 - Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events.
Rundlöf AK, Arnér ES. Rundlöf AK, et al. Antioxid Redox Signal. 2004 Feb;6(1):41-52. doi: 10.1089/152308604771978336. Antioxid Redox Signal. 2004. PMID: 14980055 Review. - Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase.
Lu J, Berndt C, Holmgren A. Lu J, et al. Biochim Biophys Acta. 2009 Nov;1790(11):1513-9. doi: 10.1016/j.bbagen.2009.04.013. Epub 2009 May 3. Biochim Biophys Acta. 2009. PMID: 19406206 Review.
Cited by
- The emerging role of the thioredoxin system in angiogenesis.
Dunn LL, Buckle AM, Cooke JP, Ng MK. Dunn LL, et al. Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2089-98. doi: 10.1161/ATVBAHA.110.209643. Epub 2010 Aug 26. Arterioscler Thromb Vasc Biol. 2010. PMID: 20798378 Free PMC article. Review. - Anticancer activity of metal complexes: involvement of redox processes.
Jungwirth U, Kowol CR, Keppler BK, Hartinger CG, Berger W, Heffeter P. Jungwirth U, et al. Antioxid Redox Signal. 2011 Aug 15;15(4):1085-127. doi: 10.1089/ars.2010.3663. Epub 2011 May 11. Antioxid Redox Signal. 2011. PMID: 21275772 Free PMC article. Review. - Maternal selenium status during early gestation and risk for preterm birth.
Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V. Rayman MP, et al. CMAJ. 2011 Mar 22;183(5):549-55. doi: 10.1503/cmaj.101095. Epub 2011 Feb 14. CMAJ. 2011. PMID: 21324870 Free PMC article. - The permissive role of mitochondria in the induction of haem oxygenase-1 in endothelial cells.
Ricart KC, Bolisetty S, Johnson MS, Perez J, Agarwal A, Murphy MP, Landar A. Ricart KC, et al. Biochem J. 2009 Apr 15;419(2):427-36. doi: 10.1042/BJ20081350. Biochem J. 2009. PMID: 19161347 Free PMC article.
References
- Oster O., Prellwitz W. Selenium and cardiovascular disease. Biol. Trace Elem. Res. 1990;24:91–103. - PubMed
- Alissa E. M., Bahijri S. M., Ferns G. A. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med. Sci. Monit. 2003;9:RA9–RA18. - PubMed
- Kuhn H., Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radical Biol. Med. 2002;33:154–172. - PubMed
- Huang H. S., Chen C. J., Suzuki H., Yamamoto S., Chang W. C. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases. Prostaglandins Other Lipid Mediators. 1999;58:65–75. - PubMed
- Schnurr K., Belkner J., Ursini F., Schewe T., Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 1996;271:4653–4658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous