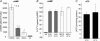Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers - PubMed (original) (raw)
Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers
Zsuzsa Bebok et al. J Physiol. 2005.
Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic AMP-regulated chloride channel. Mutations in the CFTR gene result in cystic fibrosis (CF). The most common mutation, deltaF508, results in endoplasmic reticulum-associated degradation (ERAD) of CFTR. DeltaF508 CFTR has been described as a temperature-sensitive mutation that can be rescued following growth at 27 degrees C. In order to study the processing and function of wild-type and rescued deltaF508 CFTR at the cell surface under non-polarized and polarized conditions, we developed stable cell lines expressing deltaF508 or wild-type CFTR. CFBE41o- is a human airway epithelial cell line capable of forming high resistance, polarized monolayers when cultured on permeable supports, while HeLa cells are normally grown under non-polarizing conditions. Immunoprecipitation, cell surface biotinylation, immunofluorescence, and functional assays confirmed the presence of deltaF508 CFTR at the cell surface in both cell lines after incubating the cells for 48 h at 27 degrees C. However, stimulators of wild-type CFTR such as forskolin, beta2-adrenergic or A2B-adenosine receptor agonists failed to activate rescued deltaF508 CFTR in CFBE41o- monolayers. Rescued deltaF508 CFTR could be stimulated with genistein independent of pretreatment with cAMP signalling agonists. Interestingly, rescued deltaF508 CFTR in HeLa cells could be efficiently stimulated with either forskolin or genistein to promote Cl- transport. These results indicate that deltaF508 CFTR, when rescued in CFBE41o- human airway epithelial cells, is poorly responsive to signalling pathways known to regulate wild-type CFTR. Furthermore, the differences in rescue and activation of deltaF508 CFTR in the two cell lines suggest that cell-type specific differences in deltaF508 CFTR processing are likely to complicate efforts to identify potentiators and/or correctors of the deltaF508 defect.
Figures
Figure 1. Characterization of HeLa WT, HeLa ΔF, CFBE41o– WT and CFBE41o–ΔF cell lines
A, real time RT-PCR to measure CFTR mRNA levels. CFTR transcript levels were measured using TaqMan quantitative PCR using 18S rRNA as control. Samples were internally normalized to 18S rRNA, and plotted as CFTR mRNA levels relative to 18S rRNA. Parental HeLa and CFBE41o− cells were used as controls. Mean and
s.d.
of n = 6 samples amplified under the same conditions. B, CFTR protein expression. CFTR was immunoprecipitated from 500 μg total cellular proteins using 24–1, anti-C terminal antibody, in vitro phosphorylated using [γ-32P]ATP, PKA (protein kinase A), separated on 6% PAGE and detected using Phosphorimager analysis. Calu-3 cells were used as controls for wild-type CFTR expression. B and B represents core glycosylated, ER form of CFTR. B and C represent fully glycosylated CFTR. Comparisons shown are from one day of study, and representative of > three studies in each of the conditions. C, low temperature (27°C) rescue of ΔF508 CFTR in HeLa ΔF and CFBE41o− F cells. Representative gels show the presence of B and B only (lane 1) in HeLa ΔF and CFBE41o−ΔF cells when the cells were grown at 37°C and the appearance of B and C after a 48 h incubation at 27°C (lanes 2–3). D, summary 27°C rescue experiments. Results are plotted as C/B band ratios after growing the cells at 27°C for 48 h, mean ±
s.e.m.
, n = 12 per condition (P < 0.01 for HeLa C/B ratios compared with CFBE41o− C/B ratios).
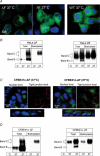
Figure 2. CFTR detection in HeLa ΔF, HeLa WT, CFBE41o–ΔF, and CFBE41o– WT cells by immunofluorescence and cell surface biotinylation
A, cell immunofluorescence studies in HeLa cells. HeLa ΔF508 CFTR cells grown at 37°C (Left) and 27°C for 48 hr (Middle). ΔF508 CFTR staining (in green) moves from perinuclear (nucleus in blue) to cytoplasmic/surface at low temperature. HeLa Wt CFTR cells grown at 37°C are shown to the right. Colour figures can be seen in the online versions, while the accompanying journal figures are provided in greyscale. B, cell biotinylation experiments in HeLa cells. Total protein input was matched (25 μg lane−1). Left, HeLa ΔF cells. Representative gels are shown with total (T) and biotinylated (B) CFTR. Biotinylated B and C is detected following growth at low temperature. Right, HeLa WT cells. Representative gels are shown with total (T) and biotinylated (B) CFTR. Biotinylated B and C is detected following growth at 27°C and 37°C. C, immunofluorescence studies in CFBE41o− monolayers. Left, CFBE41o−ΔF cells grown on permeable supports at 37°C. En face images at the nuclear level (left) and at the level of tight junctions (right). ZO-1 was stained to demonstrate well-developed tight junctions (in red). ΔF508 CFTR staining (in green) is only perinuclear at 37°C. Images below show side views of monolayers, and demonstrate ΔF508 CFTR localization to the perinuclear region. Right, CFBE41o−ΔF cells grown on permeable supports at 27°C. En face images at the nuclear level (left) and at the level of tight junctions (right). ZO-1 was stained to demonstrate well-developed tight junctions (red). After 48 h incubation at 27°C, ΔF508 CFTR staining increased and could be seen at the level of tight junctions at the apical cell surface. Images in the middle show side views of monolayers and demonstrate ΔF508 CFTR localization to the apical membrane after growth at 27°C. Colour figures can be seen in the online versions, while the accompanying journal figures are provided in greyscale. D, cell biotinylation experiments in CFBE41o− monoalyers. Total protein input was matched (25 μg lane−1). Left, CFBE41o−ΔF cells. Representative gels are shown with total (T) and biotinylated (B) CFTR. Biotinylated B and C is detected following growth at low temperature. Right, CFBE41o− WT cells. Representative gels are shown with total (T) and biotinylated (B) CFTR. Biotinylated B and C is detected following growth at 27°C > 37°C.
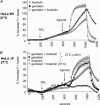
Figure 3. Halide efflux in HeLa cells
A, HeLa WT cells. Cells were grown at 37°C prior to study by SPQ as described in Methods. Each curve is the mean ±
s.e.m.
of ∼50 cells studied in the indicated condition. Halide efflux was activated by the addition of forskolin (10 μ
m
), genistein (50 μ
m
) or forkolin and genistein together at the time indicated by the arrow (agonist). Additive effects for the two agonists are demonstrated. Fluorescence was requenched in NaI buffer at the end of the experiments. B, HeLa ΔF cells grown at 27°C and 37°C. Cells were stimulated with forskolin (10 μ
m
), genistein (50 μ
m
), or forskolin + genistein indicated by the arrow (agonist). Additive effects for the two agonists are demonstrated. Cells grown at 37°C failed to respond to the combination of forskolin + genistein.
Figure 4. Cl– conductance in CFBE41o– WT monolayers
Cells were grown as monolayers at 37°C and studied in Ussing chambers as described in Methods. LoCl−, switch to apical LoCl− buffer; Am, addition of amiloride (100 μ
m
) to the apical compartment; Glyb, addition of glybenclamide (200 μ
m
) to the apical compartment. Axes are noted within each panel (μA –Y axis, time in minutes –X axis). A_–_C, examples of monolayers stimulated with forskolin (10 μ
m
, A), NECA (10 μ
m
, B), or albuterol (10 μ
m
, C). Brisk currents are produced by all three agonists, with blockade following addition of glybenclamide. D, summary of dose–response experiments performed in CFBE41o− WT monolayers. Cells were stimulated with increasing concentrations of agonists (0.1, 1.0, 10 μ
m
) as shown. Forskolin, NECA, and albuterol produced maximal currents at 0.1–1.0 μ
m
, and Ado produced maximal currents at 1.0–10 μ
m
(consistent with stimulation of the low affinity A2B AR). n = 6–8 filters studied in each condition. *NECA, ALB and Forskolin currents at 2000 s > 1000 s (P < 0.001). †Ado currents at 3000 s > 2000 s (P < 0.001).
Figure 5. Cl– conductance in parental CFBE41o– monolayers (no CFTR transduction)
Parental cells were grown as monolayers at 27°C and studied in Ussing chambers as described in Methods. LoCl−, switch to apical LoCl− buffer; Am, addition of amiloride (100 μ
m
) to the apical compartment; Glyb, addition of glybenclamide (200 μ
m
) to the apical compartment. A, cells were stimulated with 10 μ
m
agonists as indicated (forskolin, NECA, albuterol) followed by genistein (50 μ
m
). No currents were stimulated by any of the agonists (n = 6 filters/condition). B, cells were stimulated with the calcium mobilizing agents ionomycin (2 μ
m
) or A23187 (5 μ
m
) as indicated. Both agonists produced rapid spikes in current followed by sustained conductance (*P < 0.01 compared with 1000 s currents, n = 6 filters/condition).
Figure 6. Cl– conductance in CFBE41o–ΔF monolayers grown at 27°C (48 h)
Cells were grown as monolayers at 27°C and studied in Ussing chambers as described in Methods. LoCl−, switch to apical LoCl− buffer; Am, addition of amiloride (100 μ
m
) to the apical compartment; Glyb, addition of glybenclamide (200 μ
m
) to the apical compartment. Axes are noted within each panel (μA –Y axis, time in minutes –X axis). A–C, examples of monolayers stimulated with albuterol (10 μ
m
, A), NECA (10 μ
m
, B), or forskolin (10 μ
m
, C). Minimal currents are produced by all three agonists. Subsequent stimulation with genistein (50 μ
m
, apical and basolateral) produces large, sustained currents that are sensitive to glybenclamide blockade. D, summary of dose–response experiments performed in CFBE41o−ΔF508 monolayers. Cells were stimulated with increasing concentrations of agonists (0.1, 1.0, 10 μ
m
) as shown. Forskolin, NECA and albuterol produced minimal currents at all concentrations. In contrast, genistein (50 μ
m
, apical and basolateral) stimulated robust Cl− conductance that was sensitive to glybenclamide blockade (*P < 0.001 compared with at 3000 s currents for each condition). n = 8–12 filters studied in each condition.
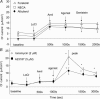
Figure 7. cAMP and ATP levels in CFBE41o–ΔF cells
For cAMP measurements, cells were grown in 60 mm dishes at 37°C or 27°C (48 h), stimulated with agonists (10 min, 37°C), and then cell cAMP was measured as described in Methods. For ATP measurements, monolayers were grown on 24 mm diameter inserts at an air–liquid interface until confluent, and placed in incubators set at 37°C or 27°C (48 h). On the day of study, monolayers were placed in 37°C incubators for 30 min (to simulate Ussing chamber conditions), and then cell ATP was measured as described in Methods. A, cAMP levels produced by 10 μ
m
forskolin, albuterol, or NECA. cAMP levels produced by forskolin, albuterol and NECA (10 μ
m
agonists) were > Controls (*P < 0.001); cAMP levels produced by forskolin and albuterol were > NECA (†P < 0.001); cAMP produced by forskolin > albuterol (‡P < 0.001). n = 4 dishes/condition. B, low temperature growth does not inhibit cAMP production. cAMP levels produced by NECA and albuterol in CFBE41o− cells grown at 37°C and 27°C (48 h). cAMP levels are normalized (%) to the mean value obtained at 37°C for the two agonists. Growth of cells at 27°C had no effect on cAMP production by either agonist (n = 4 dishes/condition). C, low temperature growth does not reduce cell ATP levels. Monolayers grown at 27°C (CFBE41o−ΔF) had similar cell ATP levels compared with CFBE41o− WT cells (n = 3 inserts/condition).
Figure 8. Genistein-stimulated Cl– conductance in CFBE41o– monolayers
Cells were grown as monolayers at 27°C (48 h) or 37°C and studied in Ussing chambers as described in Methods. LoCl−, switch to apical LoCl− buffer; Am, addition of amiloride (100 μ
m
) to the apical compartment; Glyb, addition of glybenclamide (200 μ
m
) to the apical compartment. A, no additive effects of cAMP agonists and genistein on Cl− conductance are seen in CFBE41o−ΔF monolayers. CFBE41o−ΔF cells (open symbols) were grown at 27°C (48 h) prior to study. CFBE41o− WT (filled symbols) cells were grown at 37°C. Increasing concentrations of genistein (1, 10, 50 μ
m
) were applied to activate _I_sc in NECA or albuterol pretreated (10 μ
m
) or Control (no prestimulation) CFBE41o−ΔF monolayers. H89 treated monolayers were exposed to H89-Cl (40 μ
m
) for 30 min prior to stimulation. Neither receptor agonist had additive/synergistic effects on _I_sc when combined with genistein compared with genistein alone (‘Control’), and genistein currents were sensitive to PKA blockade with H89. CFBE41o− WT cells stimulated with increasing concentrations of genistein (alone) displayed similar maximal Cl− conductance compared with CFBE41o−ΔF cells. *P < 0.001 compared with ΔF508 condition. n = 8–12 filters/condition. B, genistein stimulated _I_sc measurements in CBE41o−ΔF monolayer grown at 37°C. Parental CFBE41o− cells (no CFTR transduction) and CFBE41o−ΔF cells grown at 27°C were used as controls testing the presence of minimal levels of ΔF508 CFTR in the apical membrane of CFBE41o−ΔF cells grown at 37°C. Increasing concentrations of forskolin (0.1, 1.0, 10 μ
m
) failed to stimulate _I_sc. Small genistein stimulated currents were seen in CFBE41o−ΔF grown at 37°C (2.18 ± 0.28 μA cm−2). No currents in parental CFBE41o− cells grown at 27°C were recorded. Robust I_sc was measured in monolayers stimulated with genistein after low temperature correction. *P < 0.001 for CFBE41o−ΔF monolayers grown at 27°C versus 37°C and parental controls; †_P < 0.001 for CFBE41o−ΔF grown at 37°C compared with parental CFBE41o− cells at 27°C. n = 8–12 filters/condition.
Similar articles
- DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers.
Rowe SM, Pyle LC, Jurkevante A, Varga K, Collawn J, Sloane PA, Woodworth B, Mazur M, Fulton J, Fan L, Li Y, Fortenberry J, Sorscher EJ, Clancy JP. Rowe SM, et al. Pulm Pharmacol Ther. 2010 Aug;23(4):268-78. doi: 10.1016/j.pupt.2010.02.001. Epub 2010 Mar 10. Pulm Pharmacol Ther. 2010. PMID: 20226262 Free PMC article. - The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells.
Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, Stanton BA. Swiatecka-Urban A, et al. J Biol Chem. 2005 Nov 4;280(44):36762-72. doi: 10.1074/jbc.M508944200. Epub 2005 Aug 30. J Biol Chem. 2005. PMID: 16131493 - Novel amino-carbonitrile-pyrazole identified in a small molecule screen activates wild-type and ΔF508 cystic fibrosis transmembrane conductance regulator in the absence of a cAMP agonist.
Namkung W, Park J, Seo Y, Verkman AS. Namkung W, et al. Mol Pharmacol. 2013 Sep;84(3):384-92. doi: 10.1124/mol.113.086348. Epub 2013 Jun 20. Mol Pharmacol. 2013. PMID: 23788656 Free PMC article. - Pharmacological treatment of the ion transport defect in cystic fibrosis.
Roomans GM. Roomans GM. Expert Opin Investig Drugs. 2001 Jan;10(1):1-19. doi: 10.1517/13543784.10.1.1. Expert Opin Investig Drugs. 2001. PMID: 11116277 Review. - Protein processing and inflammatory signaling in Cystic Fibrosis: challenges and therapeutic strategies.
Belcher CN, Vij N. Belcher CN, et al. Curr Mol Med. 2010 Feb;10(1):82-94. doi: 10.2174/156652410791065408. Curr Mol Med. 2010. PMID: 20205681 Free PMC article. Review.
Cited by
- ΔF508 CFTR surface stability is regulated by DAB2 and CHIP-mediated ubiquitination in post-endocytic compartments.
Fu L, Rab A, Tang Lp, Bebok Z, Rowe SM, Bartoszewski R, Collawn JF. Fu L, et al. PLoS One. 2015 Apr 16;10(4):e0123131. doi: 10.1371/journal.pone.0123131. eCollection 2015. PLoS One. 2015. PMID: 25879443 Free PMC article. - The Ago2-miRNA-co-IP Assay to Study TGF- β1 Mediated Recruitment of miRNA to the RISC in CFBE Cells.
Mitash N, Donovan JE, Swiatecka-Urban A. Mitash N, et al. J Vis Exp. 2020 Jul 31;(161):10.3791/61571. doi: 10.3791/61571. J Vis Exp. 2020. PMID: 32894261 Free PMC article. - Mutant CFTR Drives TWIST1 mediated epithelial-mesenchymal transition.
Quaresma MC, Pankonien I, Clarke LA, Sousa LS, Silva IAL, Railean V, Doušová T, Fuxe J, Amaral MD. Quaresma MC, et al. Cell Death Dis. 2020 Oct 26;11(10):920. doi: 10.1038/s41419-020-03119-z. Cell Death Dis. 2020. PMID: 33106471 Free PMC article. - Expression of SLC26A9 in Airways and Its Potential Role in Asthma.
Ousingsawat J, Centeio R, Schreiber R, Kunzelmann K. Ousingsawat J, et al. Int J Mol Sci. 2022 Mar 10;23(6):2998. doi: 10.3390/ijms23062998. Int J Mol Sci. 2022. PMID: 35328418 Free PMC article. - Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers.
LeSimple P, Liao J, Robert R, Gruenert DC, Hanrahan JW. LeSimple P, et al. J Physiol. 2010 Apr 15;588(Pt 8):1195-209. doi: 10.1113/jphysiol.2009.182246. Epub 2010 Feb 15. J Physiol. 2010. PMID: 20156845 Free PMC article.
References
- Al-Nakkash L, Hwang TC. Activation of wild-type and ΔF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflugers Arch. 1999;437:553–561. - PubMed
- Andersson C, Servetnyk Z, Roomans GM. Activation of CFTR by genistein in human airway epithelial cell lines. Biochem Biophys Res Commun. 2003;308:518–522. - PubMed
- Bear CE, Li CH, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, Riordan JR. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. - PubMed
- Bebok Z, Venglarik CJ, Panczel Z, Jilling T, Kirk KL, Sorscher EJ. Activation of ΔF508 CFTR in an epithelial monolayer. Am J Physiol. 1998;275:C599–C607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R0-HL076587/HL/NHLBI NIH HHS/United States
- R01 DK60065/DK/NIDDK NIH HHS/United States
- R01 HL067088/HL/NHLBI NIH HHS/United States
- R01 DK060065/DK/NIDDK NIH HHS/United States
- DK54781/DK/NIDDK NIH HHS/United States
- R01-HL67088/HL/NHLBI NIH HHS/United States
- R01 HL076587/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources