The role of pericytes in blood-vessel formation and maintenance - PubMed (original) (raw)
Review
The role of pericytes in blood-vessel formation and maintenance
Gabriele Bergers et al. Neuro Oncol. 2005 Oct.
Abstract
Blood vessels are composed of two interacting cell types. Endothelial cells form the inner lining of the vessel wall, and perivascular cells--referred to as pericytes, vascular smooth muscle cells or mural cells--envelop the surface of the vascular tube. Over the last decades, studies of blood vessels have concentrated mainly on the endothelial cell component, especially when the first angiogenic factors were discovered, while the interest in pericytes has lagged behind. Pericytes are, however, functionally significant; when vessels lose pericytes, they become hemorrhagic and hyperdilated, which leads to conditions such as edema, diabetic retinopathy, and even embryonic lethality. Recently, pericytes have gained new attention as functional and critical contributors to tumor angiogenesis and therefore as potential new targets for antiangiogenic therapies. Pericytes are complex. Their ontogeny is not completely understood, and they perform various functions throughout the body. This review article describes the current knowledge about the nature of pericytes and their functions during vessel growth, vessel maintenance, and pathological angiogenesis.
Figures
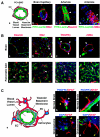
Fig. 1
Pericytes A. Capillaries are composed of endothelial cells (ECs; green) that form the inner lining of the wall with a surrounding basal lamina and pericytes (PC; red) that extend long cytoplasmic processes over the surface of the vascular tube (a, b). Larger vessels have several layers of pericytes and smooth muscle cells. Arterioles have strong elastic vessel walls (c) with dense layers of concentrically formed smooth muscle cells (d) to withstand the blood pressure. B. Immunohistochemical detection of pericytes exemplified in murine brain (a–d; red) and pancreatic islet tissues (e–h; red). NG2 is expressed in pericytes (b; white arrow), but also in microglia cells (b; yellow arrow) in the brain, whereas NG2 appears to be solely specific for pericytes in the pancreas (f; white arrow). α-SMA+-pericytes (h) are less abundant in the pancreatic islets than NG2+ or desmin+ cells (e, f). Green arrows indicate EC and white arrows mark PC, while the yellow arrow points to microglia cells. Blood vessels in the tissues were visualized with FITC-labeled tomato lectin (green), and tissue sections were stained with red-labeled antibodies for desmin (A.c; B.a, e), NG2 (A.b; B.b, f), PDGFRβ (B.c, g), or α-SMA (A.d; B.d, h) to visualize pericytes and incubated with DAPI to identify nuclei. All pictures were captured on a confocal microscope at 63× magnification (A.b–d; B.a–h) with an additional 3× zoom (B.c, d) and additional 8× zoom (A.b). C. Brain capillaries consist of a continuous endothelium with complex tight junctions and interact with astrocytic foot processes and pericytes to constitute the BBB (a). Antibodies for glial fibrillary acidic protein (GFAP) detect astrocytes with their cytoplasmic processes in red (b–e), and antibodies for PDGFRβ identify pericytes in blue (white arrows; b, c). ECs are stained with both FITC-labeled tomato lectin (Lycopersicon esculentum) and FITC-labeled CD31 in green (b–e). Glioblastoma cells (in blue; cyan arrows) invade the brain parenchyma and migrate along blood vessels interrupting the interaction of normal astrocytes and the vasculature. Yellow arrows point to astrocytic foot processes; cyan arrows indicate glioblastoma cells and white arrows show pericytes. Pictures were captured on a confocal microscope at 63× magnification with an additional 4× zoom.
Fig. 2
Pericytes in vasculogenesis and angiogenesis A. Endothelial cells (ECs) and pericytes/vSMCs (PC) arise from different precursor cells. ECs develop from angioblasts or hemangioblasts in the embryo, while pericytes/vSMCs are derived form mesenchymal stem cells or neurocrest cells. In vitro data indicate that there exists a common vascular progenitor derived from embryonic stem cells that can give rise to EC in the presence of VEGF, and to PC in the presence of PDGF-B. In the embryo, endothelial cells first assemble into a simple capillary network. Vessels then sprout and prune (angiogenesis, B), become stabilized by pericytes/vSMCs that are recruited by PDGF-B-secreting endothelial cells, and segregate into the different vessel types. Arterioles exhibit a high density of circumferentially oriented SMCs and thicker EC walls to withstand the blood pressure. Venules, like capillaries, have irregularly arranged pericytes with multiple cytoplasmic processes and are composed of thinner EC walls with valves to prevent backflow of blood. B. New vessels are formed from existing blood vessels by endothelial cell bridging, intussusceptions, and/or sprouting. This is in general preceded by pericyte detachment from the vessel wall and subsequent vessel hyperdilation. When vessels form new sprouts, the vascular basement membrane is first degraded to enable EC to move into the ECM. This is accompanied by EC proliferation and migration toward an angiogenic stimulus. ECs can be either guided by EC tip cells expressing high levels of PDGF-B or by pericytes. Immature, newly formed vessels cease the proliferation, and ECs adhere to each other, form a lumen and become encircled by a basement membrane with recruited pericytes. ECs, and also PCs, can be recruited from the bone marrow, specifically in tumor angiogenesis. Green arrows indicate EC; white arrows point to PC.
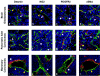
Fig. 3
Pericytes in tumors. Tumor sections from three mouse models of tumorigenesis were used to visualize pericytes in glioblastomas (a–d), pancreatic islet carcinomas (e–h), and mammary carcinomas. SV40 Tag/H-ras transformed astrocytes intracranially injected into athymic mice, generate glioblastomas (Blouw et al. 2003). Transgenic mice expressing SV40Tag under the control of the insulin promoter develop pancreatic islet carcinomas (Hanahan, 1984). MMTV-neu mice overexpress the neu-oncogene under the control of the MMTV promoter and develop mammary adenocarcinomas (Guy et al., 1992). Blood vessels in the tumors were visualized by FITC-labeled tomato lectin (lycopersicon esculentum) and immunostaining with an FITC-labeled CD31 antibody. Tumor tissue sections were then stained with red-labeled antibodies for desmin (a, e, i), NG2 (b, f, j), PDGFRβ (c, g, k), or α-SMA (d, h, l) and incubated with DAPI to identify nuclei. Tumor vessels (green) are very distinct from their normal counterparts (see Fig. 1) because they become irregularly shaped, hyperdilated, and enlarged. In addition, tumor vessels differ among tumors. The vasculature of mammary carcinomas is enormously enlarged and thickened, GBM vessels are thin and hyperdilated, and vascular tubes in islet carcinomas are more irregular but only slightly hyperdilated. Pericyte coverage of blood vessels is also tumor dependent. Islet carcinomas have more pericyte coverage than GBMs or mammary carcinomas. Tumor pericytes are in general more loosely attached (c, d, f, h, i, k; white asterisks), and in some tumors like GBM less abundant (a, b) when compared to normal tissue. In addition, other tumors like mammary carcinomas contain clusters of pericytes that appear not to be distributed properly (i, l). Pericytes in tumors intend to bridge between blood vessels and to extend their cellular processes toward tumor cells (h, i, l).
Similar articles
- Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine.
Ahmed TA, El-Badri N. Ahmed TA, et al. Adv Exp Med Biol. 2018;1079:69-86. doi: 10.1007/5584_2017_138. Adv Exp Med Biol. 2018. PMID: 29282647 Review. - Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche.
Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L Jr. Díaz-Flores L, et al. Histol Histopathol. 2009 Jul;24(7):909-69. doi: 10.14670/HH-24.909. Histol Histopathol. 2009. PMID: 19475537 Review. - Pericytes and vessel maturation during tumor angiogenesis and metastasis.
Raza A, Franklin MJ, Dudek AZ. Raza A, et al. Am J Hematol. 2010 Aug;85(8):593-8. doi: 10.1002/ajh.21745. Am J Hematol. 2010. PMID: 20540157 Review. - Pericytes and ocular diseases.
Motiejūnaite R, Kazlauskas A. Motiejūnaite R, et al. Exp Eye Res. 2008 Feb;86(2):171-7. doi: 10.1016/j.exer.2007.10.013. Epub 2007 Nov 5. Exp Eye Res. 2008. PMID: 18078933 Review. - Pericytes: developmental, physiological, and pathological perspectives, problems, and promises.
Armulik A, Genové G, Betsholtz C. Armulik A, et al. Dev Cell. 2011 Aug 16;21(2):193-215. doi: 10.1016/j.devcel.2011.07.001. Dev Cell. 2011. PMID: 21839917 Review.
Cited by
- The Role of Pericytes in Inner Ear Disorders: A Comprehensive Review.
Maniaci A, Briglia M, Allia F, Montalbano G, Romano GL, Zaouali MA, H'mida D, Gagliano C, Malaguarnera R, Lentini M, Graziano ACE, Giurdanella G. Maniaci A, et al. Biology (Basel). 2024 Oct 8;13(10):802. doi: 10.3390/biology13100802. Biology (Basel). 2024. PMID: 39452111 Free PMC article. Review. - Anti-angiogenic and anti-tumor effects of TAK-593, a potent and selective inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptor tyrosine kinase.
Awazu Y, Mizutani A, Nagase Y, Tsuchiya S, Nakamura K, Kakoi Y, Kitahara O, Takeuchi T, Yamasaki S, Miyamoto N, Iwata H, Miki H, Imamura S, Hori A. Awazu Y, et al. Cancer Sci. 2013 Apr;104(4):486-94. doi: 10.1111/cas.12101. Epub 2013 Feb 18. Cancer Sci. 2013. PMID: 23305239 Free PMC article. - Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis.
Campanholle G, Ligresti G, Gharib SA, Duffield JS. Campanholle G, et al. Am J Physiol Cell Physiol. 2013 Apr 1;304(7):C591-603. doi: 10.1152/ajpcell.00414.2012. Epub 2013 Jan 16. Am J Physiol Cell Physiol. 2013. PMID: 23325411 Free PMC article. Review. - LY2228820 dimesylate, a selective inhibitor of p38 mitogen-activated protein kinase, reduces angiogenic endothelial cord formation in vitro and in vivo.
Tate CM, Blosser W, Wyss L, Evans G, Xue Q, Pan Y, Stancato L. Tate CM, et al. J Biol Chem. 2013 Mar 1;288(9):6743-53. doi: 10.1074/jbc.M112.425553. Epub 2013 Jan 18. J Biol Chem. 2013. PMID: 23335506 Free PMC article. - Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression.
Ngo MT, Harley BAC. Ngo MT, et al. Biomaterials. 2020 Oct;255:120207. doi: 10.1016/j.biomaterials.2020.120207. Epub 2020 Jun 14. Biomaterials. 2020. PMID: 32569868 Free PMC article. Review.
References
- Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. - PubMed
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neo-vascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. - PMC - PubMed
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52:127–142. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources