Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies - PubMed (original) (raw)
Comparative Study
Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies
Makoto Higuchi et al. J Neurosci. 2005.
Abstract
Abundant filamentous tau inclusions in oligodendrocytes (OLGs) are hallmarks of neurodegenerative tauopathies, including sporadic corticobasal degeneration and hereditary frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17). However, mechanisms of neurodegeneration in these tauopathies are unclear in part because of the lack of animal models for experimental analysis. We address this by generating transgenic (Tg) mice expressing human tau exclusively in OLGs using the 2',3'-cyclic nucleotide 3'-phosphodiesterase promoter. Filamentous OLG tau inclusions developed in these Tg mice as a result of human tau expression in OLGs, especially those expressing the FTDP-17 human P301L mutant tau. Notably, structural disruption of myelin and axons preceded the emergence of thioflavin-S positive tau inclusions in OLGs, but impairments in axonal transport occurred even earlier, whereas motor deficits developed subsequently, especially in Tg mice with the highest tau expression levels. These data suggest that the accumulation of tau in OLG cause neurodegeneration, and we infer they do so by disrupting axonal transport. We suggest that similar defects may also occur in sporadic and hereditary human tauopathies with OLG tau pathologies.
Figures
Figure 1.
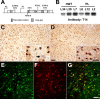
Expression of Tg human tau in OLGs of the murine nervous system. A, Schematic representation of the transgene construct used to generate tau Tg mice, which consists of a stop codon, human T34 with or without the PL mutation, and polyadenylation site (Poly-[A]) inserted into _Hin_dIII site between CNP exons 0 and 1 (E0 and E1). Transgene expression in OLGs was directed by the CNP promoter P1, whereas expression from CNP promoter P2 was impeded by a stop codon upstream of the T34 sequence. B, Immunoblots using the human tau-specific anti-tau antibody T14 demonstrate varying levels of transgene expression in the brains of the different tau Tg lines. All protein samples were derived from 3-month-old heterozygous Tg mice. C, D, Sections of the basal ganglia (C) and spinal cord (D) of a 3-month-old line 12 PL Tg mouse immunolabeled with antibody 17026 show intense tau immunoreactivity in glial cells with the morphology of OLGs, whereas neurons show far less intense positivity for endogenous mouse tau (arrow). E-G, Double-immunofluorescence staining with anti-CNP antibody (E) and anti-tau antibody 17026 (F) in spinal cord of a 3-month-old line 12 PL Tg mouse showing robust expression of tau in CNP-positive OLGs. A dualchannel image (G) indicates ∼100% colocalization of CNP and tau positivity in OLGs. Scale bars: C, D, 100 μm; insets in C, D, 20 μm; E-G, 50 μm.
Figure 2.
Formation of fibrillary human tau inclusion in OLGs of PL Tg mice as a function of age. A, T14 immunoblots of human tau in HS-, RIPA-, and FA-soluble fractions extracted from the brains (top row) and spinal cords (bottom row) of line 39 hWT and line 12 PL Tg mice at different ages. Levels of HS-soluble tau remained stable during aging, as was the case for RIPA- and FA-extractable tau proteins in the hWT Tg mice, but there was a pronounced increase in these relatively insoluble species of tau in older PL Tg mice. The retarded electrophoretic mobility of the FA-extractable tau in older PL Tg mice is consistent with its increased phosphorylation. B-D, Fibrillary lesions in presumptive OLGs in a Gallyas silver-stained spinal cord section of 15-month-old line 12 PL Tg mouse. High-power photomicrographs of the inclusions in the anterior horn (C) and anterior column (D) reveal that pathological fibrillar tau extends into proximal processes of OLGs. E, Similar OLG inclusion labeled with thioflavin-S [counterstained for nuclei with 4′,6′-diamidino-2-phenylindole (DAPI)] in 15-month-old line 12 PL Tg mouse. F, Gallyas silver-positive coiled bodies in the frontal white matter of a patient with CBD, showing resemblance of fibrillary lesions in the PL Tg mice to those in FTD patients. Scale bars: B, 50 μm; C-F, 20 μm.
Figure 3.
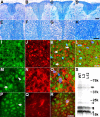
Progressive degeneration of OLGs and axons in the spinal cord of PL Tg mice. A-H, Age-associated loss of myelin in the posterior column of the spinal cord revealed by Luxol fast blue staining on 6- (A, E), 9- (B, F), and 12- (C, G) month-old PL Tg and 12-month-old WT non-Tg mice (D, H). High-power view photomicrographs are displayed in the bottom panels (E-H). I-L, Disrupted myelin accompanies axonal damage in the posterior column of the spinal cord. Double-immunofluorescence staining of a frozen section from 6-month-old PL Tg mouse using anti-CNP (I) and anti-NFH (RMO24) (J) antibodies demonstrates disorganization of myelin and atrophy or loss of axons as indicated by arrows on a merged image (K; counterstained with DAPI), whereas a WT non-Tg mouse (L) at the same age does not show these pathological alterations. M-R, Impaired kinesin transport in spinal cord OLGs of PL Tg mouse at 9 months of age. Photomicrographs of doubly stained OLGs of an age-matched WT mouse with channels for kinesin (M), CNP (N), and merged images (O; counterstained with DAPI) depict normal physiological distribution of kinesin that ranges from the cell body to distal processes (arrow in M) in a CNP-positive OLG. Arrowhead in M indicates kinesin in the axon. Unlike WT mice, PL Tg mouse showed reduced kinesin staining in distal processes of OLGs (arrow in P), which were filled with tau immunoreactivity (Q). Multichannel photomicrograph demonstrates disrupted kinesin staining in tau-positive OLGs (R; counterstained with DAPI). S, Loss of myelin components in the brains of 12-month-old PL Tg mice visualized by Coomassie blue. Myelin samples extracted from line 2 and line 12 mice contained significantly less myelin constituents, including MBP (arrowheads) and CNP (arrows), than age-matched WT mouse-derived sample. Other minor bands are also less intense in the PL Tg mice than those in the WT mouse, implying uniform reduction of myelin proteins as a result of OLG tau pathology. Scale bars: A-D, 200 μm; E-H, 100 μm; I-L, P-R, 20 μm.
Figure 4.
Ultrastructural alterations of myelin and axons associated with accumulation of abnormal tau filaments in the spinal cords of the line 12 PL Tg mice at 12 months of age. A-C, Myelin pathology and axonal degeneration demonstrated by TEM. Although the WT mouse did not develop changes in myelin and axons (A), myelin in the PL mouse is fragmented and disorganized (arrows in B), and the axons within the myelin sheaths are severely atrophic. Disruption of myelin and axon loss are shown at higher magnification (C). D-F, Accumulation of tau-immunoreactive filaments in the PL Tg mouse revealed by immuno-EM with anti-tau antibody 17026. Abnormal filaments were present between detached myelin layers (D) and in areas apposed to degenerating myelin lacking a central axon (arrow in D). High-power view shows intense labeling of well oriented filaments with anti-tau antibody (E). Tau-immunolabeled filaments were also localized to OLG processes (arrow in F), whereas filaments in axons were not tau positive and had side arms characteristic of NFs (arrowhead in F). Scale bars: A, B, 2 μm; C, D, 1 μm; E, F, 500 nm.
Figure 5.
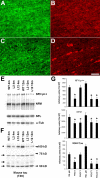
Biochemical impact of OLG tau accumulations on axons in the optic nerve of the PL Tg mice. A, Overexpression of Tg tau in the optic nerve interfascicular OLGs shown by double-immunofluorescence staining with anti-CNP antibody (A, C) and anti-tau antibody 17026 (B, D) for 6-month-old WT non-Tg (A, B) and line 12 PL tau Tg (C, D) mice. In contrast to diffuse tau immunoreactivity in WT OLGs, strong tau staining is seen in specific CNP-positive OLGs of the PL Tg mouse. CNP staining in myelin and tau staining in axons were moderately diminished in the PL Tg mouse compared with the WT mouse. E-G, Immunoblotting for optic nerve samples with antibodies against phosphorylated NFH (NFH p++), NFM, NFL, α-tubulin (α-tub) (E), and endogenous mouse tau (F), and quantification of the blots (n = 4 in each group) (G). Substantial reduction of NFH and NFM occurred in the line 2 and line 12 PL Tg mice at 4 months of age (E and top and middle panels in G). The 12-month-old PL Tg mice also showed slight decrease in the amounts of NFL and α-tubulin. There were no remarkable differences in the levels of low-molecular-weight tau isoforms ranging from 50 to 70 kDa (indicated by arrows) between the WT and PL Tg mice, whereas middle-molecular-weight tau (∼100 kDa, indicated by an asterisk) in the PL Tg mice was pronouncedly reduced relative to the WT mice (F and bottom panel in G). *p < 0.05 by multiple comparison by ANOVA. Scale bar: A-D, 50 μm.
Figure 6.
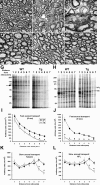
Impaired axonal transport in the optic nerves of the PL Tg mice before ultrastructural disruption of myelin and axons. A-F, TEM of the optic nerve sections of line 12 PL mice at 6 (A) and 9 (B, C) months of age, WT mice at 6 (D) and 9 (E) months, and line 39 h WT tau Tg mouse at 9 months (F). The 6-month-old PL tau Tg mouse exhibited relatively normal myelin sheaths and axons morphologically (A), whereas there were marked losses of these structures and expansion of interstitial space in the 9-month-old PL Tg mice (B). Severe disorganization of myelin and axonal atrophy are also shown in a high-power view (C). The WT and hWT Tg mice did not develop overt pathologies until 9 months of age (D-F). G-L, Quantitative assays of fast (G, I, J) and slow (H, K, L) axonal transport in the optic nerve. SDS-PAGE of protein samples from optic nerve segments indicates significant retardation of fast axonal transport in 6-month-old line 12 PL Tg mice compared with age-matched WT mice (G, I). This deficit became greater at 9 months of age (J). The arrow in G identifies a major protein band undergoing fast axonal transport and is used here as a marker for the quantification of fast transport. Slow axonal transport of proteins such as tubulin and NFL (corresponding bands are indicated in H) were also significantly slower in the 6-month-old PL Tg mice relative to the WT mice (K, L). Error bars in I-L represent SE (n = 3 in each group). *p < 0.05 by t test. Scale bars: A, B, D-F, 2 μm; C, 1 μm.
Figure 7.
Weight loss and progressive motor deficits in the PL Tg mice. A, Weights of different lines of PL and hWT Tg mice at 12 months of age. Values are normalized by the mean body weight of the WT mice at the same age. Homozygotes of the line 7 PL Tg mice are not included, because most of them did not survive until this age. n = 6 in each group. B, Frequency of limb twitching in tail suspension test. Although the hWT Tg mice (left) showed this pathologic motor phenotype at a frequency that was higher than that of WT mice reported previously (Higuchi et al., 2002a), the frequency was further augmented in the PL mice (right). The emergence of limb twitching was approximately proportional to the dosage of the transgenes in both of the hWT and PL Tg mice.
Figure 8.
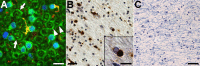
Oligodendrocytic tau accumulation and disruption of myelin sheaths in patients with FTD. A, Frozen section of the frontal white matter from a PSP patient immunolabeled with antibodies against CNP (green) and tau (PHF1, red) and counterstained with DAPI (blue). Myelin sheaths that were connected to a phospho-tau-immunoreactive OLG showed marked disorganization (arrows), although a large portion of myelin appeared intact (arrowhead). B, C, Paraffin sections of the frontal white matter from an FTD patient stained with PHF1 (B) and Luxol fast blue (C). Tau-positive inclusions were frequently found in putative OLGs (B). Round, well bordered deposits were localized to the cytoplasm of OLG as revealed in high-power photomicrograph (inset in B). Severe loss of myelin was also observed in the same region (C). Scale bars: A, inset in B, 15 μm; B, 25 μm; C, 100 μm.
Similar articles
- Transgenic mouse model of tauopathies with glial pathology and nervous system degeneration.
Higuchi M, Ishihara T, Zhang B, Hong M, Andreadis A, Trojanowski J, Lee VM. Higuchi M, et al. Neuron. 2002 Aug 1;35(3):433-46. doi: 10.1016/s0896-6273(02)00789-4. Neuron. 2002. PMID: 12165467 - Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration.
Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ. Forman MS, et al. J Neurosci. 2005 Apr 6;25(14):3539-50. doi: 10.1523/JNEUROSCI.0081-05.2005. J Neurosci. 2005. PMID: 15814784 Free PMC article. - Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation.
Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Lin WL, et al. Am J Pathol. 2003 Jan;162(1):213-8. doi: 10.1016/S0002-9440(10)63812-6. Am J Pathol. 2003. PMID: 12507904 Free PMC article. - Analysis of tauopathies with transgenic mice.
Hutton M, Lewis J, Dickson D, Yen SH, McGowan E. Hutton M, et al. Trends Mol Med. 2001 Oct;7(10):467-70. doi: 10.1016/s1471-4914(01)02123-2. Trends Mol Med. 2001. PMID: 11597522 Review. - Tau alteration and neuronal degeneration in tauopathies: mechanisms and models.
Brandt R, Hundelt M, Shahani N. Brandt R, et al. Biochim Biophys Acta. 2005 Jan 3;1739(2-3):331-54. doi: 10.1016/j.bbadis.2004.06.018. Biochim Biophys Acta. 2005. PMID: 15615650 Review.
Cited by
- The benefits and limitations of animal models for translational research in neurodegenerative diseases.
Jucker M. Jucker M. Nat Med. 2010 Nov;16(11):1210-4. doi: 10.1038/nm.2224. Epub 2010 Sep 21. Nat Med. 2010. PMID: 21052075 - The many faces of tau.
Morris M, Maeda S, Vossel K, Mucke L. Morris M, et al. Neuron. 2011 May 12;70(3):410-26. doi: 10.1016/j.neuron.2011.04.009. Neuron. 2011. PMID: 21555069 Free PMC article. Review. - Structural abnormalities in the cortex of the rTg4510 mouse model of tauopathy: a light and electron microscopy study.
Ludvigson AE, Luebke JI, Lewis J, Peters A. Ludvigson AE, et al. Brain Struct Funct. 2011 Mar;216(1):31-42. doi: 10.1007/s00429-010-0295-4. Epub 2010 Dec 9. Brain Struct Funct. 2011. PMID: 21152933 Free PMC article. - miR-142-3p regulates cortical oligodendrocyte gene co-expression networks associated with tauopathy.
Hinman JD, Ngo KJ, Kim D, Chen C, Abraham CR, Ghanbari M, Ikram MA, Kushner SA, Kawaguchi R, Coppola G, Goth K, Bellusci S, Hernandez I, Kosik KS, Fogel BL. Hinman JD, et al. Hum Mol Genet. 2021 Mar 25;30(1):103-118. doi: 10.1093/hmg/ddaa252. Hum Mol Genet. 2021. PMID: 33555315 Free PMC article. - Cell-specific MAPT gene expression is preserved in neuronal and glial tau cytopathologies in progressive supranuclear palsy.
Forrest SL, Lee S, Nassir N, Martinez-Valbuena I, Sackmann V, Li J, Ahmed A, Tartaglia MC, Ittner LM, Lang AE, Uddin M, Kovacs GG. Forrest SL, et al. Acta Neuropathol. 2023 Sep;146(3):395-414. doi: 10.1007/s00401-023-02604-x. Epub 2023 Jun 24. Acta Neuropathol. 2023. PMID: 37354322 Free PMC article.
References
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci 22: 9340-9351. - PMC - PubMed
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11: 153-163. - PubMed
- Bjartmar C, Yin X, Trapp BD (1999) Axonal pathology in myelin disorders. J Neurocytol 28: 383-395. - PubMed
- Brady S (1985) Axonal transport methods and applications. In: Neuromethods, general neurochemical techniques (Boulton A, Baker G, eds), pp 419-476. Clifton, NJ: Humana.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous