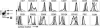A two-step process for cytokine production revealed by IL-4 dual-reporter mice - PubMed (original) (raw)
A two-step process for cytokine production revealed by IL-4 dual-reporter mice
Katja Mohrs et al. Immunity. 2005 Oct.
Abstract
To monitor IL-4 expression at the single-cell level, we generated mice with insertions of different reporter genes into both copies of the Il4 gene that permitted the simultaneous analysis of IL-4 transcripts via GFP and IL-4 protein secretion by use of huCD2. Innate and adaptive cells competent for IL-4 production were marked by GFP, while cells that presently or recently secreted IL-4 additionally displayed huCD2. After challenge with the strictly enteric helminth, Heligmosomoides polygyrus, GFP-positive innate and adaptive cells disseminated widely, but IL-4 secretion was predominantly mediated by CD4+ T cells in the intestines and draining lymphoid organs. IL-4-competent cells persisted in cured animals, and memory responses reflected rapid cytokine production at the site of rechallenge. These data reveal a two-step process for cytokine production: the first generating poised cells that disseminate systemically and the second inducing the rapid production of the cytokine in response to local stimulation.
Figures
Figure 1. Schematic of 4get/KN2 IL-4 dual-reporter mice
In heterozygous 4get/KN2 IL-4 dual-reporter mice one Il4 allele is marked by the addition of the bicistronic IRES-GFP reporter (4get). The first two exons of the other allele are replaced with the huCD2 reporter (KN2) cassette. 4get/KN2 mice have a functional copy of IL-4 as part of the bicistronic 4get allele. Filled boxes with numbers indicate exons. See Supplementary Fig. 1 for details.
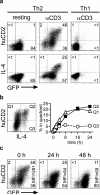
Figure 2. huCD2 expression faithfully reflects IL-4 protein secretion
CD4+ T cells were purified from naïve 4get/KN2 mice and stimulated in the presence of APC under Th2 (IL-4 + anti-IFN-γ) or Th1 (IL-12 + anti-IL-4) polarizing conditions. (a) After 6 days these cultures were transferred to uncoated (resting) or anti-CD3ε-coated (αCD3) wells and analyzed the next day for huCD2 expression (top panel) or IL-4 secretion (bottom panel) using a cytokine secretion assay. (b) The plate-bound anti-CD3ε stimulation of Th2 polarized cultures was abruptly terminated by transfer to fresh, uncoated wells (0 h) and the cultures were analyzed 24 and 48 h later. Vertical numbers indicate the MFI of GFP+/huCD2+ population. (c) Th2 cultures were generated and restimulated as in (a) for the indicated periods of time (right panel). huCD2 expression and IL-4 secretion were determined as in (a). Representative FACS plot depicts the 8 h time point. Q1: IL-4-/huCD2+; Q2: IL-4+/huCD2+; Q3: IL-4+/huCD2-. All huCD2+ and/or IL-4+ cells were also GFP+ (see a, b and data not shown).
Figure 3. huCD2 expression reflects IL-4 protein secretion by lymphoid and nonlymphoid cells in vivo
(a) Naïve 4get/KN2 mice or 4get/+ littermate controls were injected i.v. with either PBS (0 min) or anti-CD3ε. Splenic NK T cells (NK1.1+/CD4+) or conventional (NK1.1-/CD4+) CD4+ T cells were analyzed 45 and 90 min later for the expression of GFP and huCD2. (b) Naïve 4get/KN2 mice were either injected with PBS or were sensitized by i.p. injection of anti-DNP mouse IgE. 24 h later the sensitized animals were injected i.p. with either PBS or DNP30-40-HSA. Basophils (FcεRI+/CD4-/SSClo) in the livers of all groups were analyzed 2 h later for the expression of GFP and huCD2.
Figure 4. GFP and huCD2 expression in response to infection with H. polygyrus
4get/KN2 mice were infected with H. polygyrus and analyzed 2 weeks later. (a) CD4+ T cells in the indicated organs were analyzed directly ex vivo for the expression of GFP and huCD2. (b) _Hp_-infected animals were injected i.p. with PBS (PBS), anti-CD3ε (αCD3), anti-IgE (αIgE), IL-3 + IL-18 or worm extract and sacrificed 2-4 h later. CD4+ T cells, basophils (FcεRI+/CD4-/SSClo), and eosinophils (CCR3+/CD4-/SSChi) in the liver were analyzed for the expression of GFP and huCD2. (c) Peritoneal exudate cells were cultured in the absence (no extract) or presence of crude extracts prepared from adult worm (worm extract), larvae extract or S. mansoni egg antigen (SmEA) (top panel). The next day CD4+ T cells were analyzed for the expression of GFP and huCD2. The same procedure was performed after mice were immunized 1 week earlier i.p. with S. mansoni eggs (bottom panel).
Figure 5. Phenotype of huCD2+ and huCD2- GFP+ Th2 cells
4get/KN2 mice were infected with H. polygyrus and the mesLN were analyzed 2 weeks later. CD4+ T cells with a GFP-/huCD2-, GFP+/huCD2-, or GFP+/huCD2+ phenotype (as indicated on the small dot plot to the left) were analyzed for the expression of the indicated surface markers (depicted in alphanumerical order).
Figure 6. Cytokine transcripts and protein production by huCD2+ and huCD2- GFP+ Th2 cells
4get/KN2 mice were infected with H. polygyrus and the mesLN were analyzed 2 weeks later. (a) CD4+ T cells with a GFP-/huCD2-, GFP+/huCD2-, or GFP+/huCD2+ phenotype were sorted from the mesLN. (b) mRNA was immediately prepared from the respective populations, reversed transcribed and analyzed by real-time RT-PCR for the indicated transcripts normalized to GAPDH. Depicted is the expression relative to GFP- cells. Shown are the mean± SD from triplicate samples. (c) The sorted populations (a) were cultured in the absence (0 h) or presence of plate-bound anti-CD3ε. Supernatants were harvested after 4 and 24 h and analyzed by ELISA for the indicated cytokines. The detection limits are indicated by the dotted line. Depicted are the mean± SD. The stimulated CD4+ T cells (c) were analyzed after 4 and 24 h for the expression of huCD2 (d) and GFP versus huCD2 after 24 h (e). (f) The sorted populations (a) were either directly stimulated for 4 h with PMA + ionomycin (P+I, left panel) or rested for 18 h prior to 24 h stimulation on plate-bound anti-CD3 (rested, right panel) and the supernatants were analyzed by ELISA for IL-4.
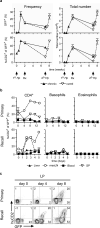
Figure 7. huCD2 expression is highly regulated during the course of primary and challenge Hp infection
(a) 4get/KN2 mice were infected with H. polygyrus (1° Hp) and CD4+ T cells in the mesLN were analyzed at the indicated time points for GFP (upper panels) and huCD2 (lower panels) expression. One cohort was drug-cured (Rx) after 2 weeks while the control group remained untreated. Animals from both groups were analyzed 5 weeks after drug treatment (7 weeks after 1° Hp). The remaining drug-cured animals were rechallenged (2° Hp) 5 weeks after drug treatment and analyzed at the indicated time points. Depicted are the mean± SD. (b) Naïve (primary) or drug-cured (recall) 4get/KN2 mice were infected with H. polygyrus and CD4+ Th2 cells (CD4+/FcεRI-/GFP+), basophils (FcεRI+/CD4-/GFP+), and eosinophils (CCR3+/GFP+) were analyzed at various time points in the indicated organs for huCD2 expression. (c) Naïve (primary) or drug-cured (recall) 4get/KN2 mice were infected with H. polygyrus and CD4+ T cells in the LP were analyzed for the expression of GFP and huCD2 at the indicated days.
Similar articles
- Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine.
Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF Jr, Elliott DE, Weinstock JV. Setiawan T, et al. Infect Immun. 2007 Sep;75(9):4655-63. doi: 10.1128/IAI.00358-07. Epub 2007 Jul 2. Infect Immun. 2007. PMID: 17606601 Free PMC article. - CD40-mediated stimulation contributes to lymphocyte proliferation, antibody production, eosinophilia, and mastocytosis during an in vivo type 2 response, but is not required for T cell IL-4 production.
Lu P, Urban JF, Zhou XD, Chen SJ, Madden K, Moorman M, Nguyen H, Morris SC, Finkelman FD, Gause WC. Lu P, et al. J Immunol. 1996 May 1;156(9):3327-33. J Immunol. 1996. PMID: 8617957 - CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response.
Gause WC, Chen SJ, Greenwald RJ, Halvorson MJ, Lu P, Zhou XD, Morris SC, Lee KP, June CH, Finkelman FD, Urban JF, Abe R. Gause WC, et al. J Immunol. 1997 May 1;158(9):4082-7. J Immunol. 1997. PMID: 9126966 - A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3.
Svetić A, Madden KB, Zhou XD, Lu P, Katona IM, Finkelman FD, Urban JF Jr, Gause WC. Svetić A, et al. J Immunol. 1993 Apr 15;150(8 Pt 1):3434-41. J Immunol. 1993. PMID: 8468481 - The initial response of CD4+ IL-4-producing cells.
Xin J, Ohmori K, Nishida J, Zhu Y, Huang H. Xin J, et al. Int Immunol. 2007 Mar;19(3):305-10. doi: 10.1093/intimm/dxl147. Epub 2007 Jan 30. Int Immunol. 2007. PMID: 17267416
Cited by
- Distinct activation thresholds of human conventional and innate-like memory T cells.
Slichter CK, McDavid A, Miller HW, Finak G, Seymour BJ, McNevin JP, Diaz G, Czartoski JL, McElrath MJ, Gottardo R, Prlic M. Slichter CK, et al. JCI Insight. 2016 Jun 2;1(8):e86292. doi: 10.1172/jci.insight.86292. JCI Insight. 2016. PMID: 27331143 Free PMC article. - Naive T-cell receptor transgenic T cells help memory B cells produce antibody.
Duffy D, Yang CP, Heath A, Garside P, Bell EB. Duffy D, et al. Immunology. 2006 Nov;119(3):376-84. doi: 10.1111/j.1365-2567.2006.02446.x. Immunology. 2006. PMID: 17067314 Free PMC article. - Nematode-Infected Mice Acquire Resistance to Subsequent Infection With Unrelated Nematode by Inducing Highly Responsive Group 2 Innate Lymphoid Cells in the Lung.
Yasuda K, Adachi T, Koida A, Nakanishi K. Yasuda K, et al. Front Immunol. 2018 Sep 19;9:2132. doi: 10.3389/fimmu.2018.02132. eCollection 2018. Front Immunol. 2018. PMID: 30283458 Free PMC article. - BATF acts as an essential regulator of IL-25-responsive migratory ILC2 cell fate and function.
Miller MM, Patel PS, Bao K, Danhorn T, O'Connor BP, Reinhardt RL. Miller MM, et al. Sci Immunol. 2020 Jan 10;5(43):eaay3994. doi: 10.1126/sciimmunol.aay3994. Sci Immunol. 2020. PMID: 31924686 Free PMC article. - A mechanism for the initiation of allergen-induced T helper type 2 responses.
Sokol CL, Barton GM, Farr AG, Medzhitov R. Sokol CL, et al. Nat Immunol. 2008 Mar;9(3):310-8. doi: 10.1038/ni1558. Nat Immunol. 2008. PMID: 18300366 Free PMC article.
References
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
- Ansel KM, Greenwald RJ, Agarwal S, Bassing CH, Monticelli S, Interlandi J, Djuretic IM, Lee DU, Sharpe AH, Alt FW, Rao A. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nat Immunol. 2004;5:1251–1259. - PubMed
- Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. - PubMed
- Bix M, Locksley RM. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281:1352–1354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI45666/AI/NIAID NIH HHS/United States
- P01 AI046530/AI/NIAID NIH HHS/United States
- AI046530/AI/NIAID NIH HHS/United States
- R01 AI026918/AI/NIAID NIH HHS/United States
- P01 AI045666/AI/NIAID NIH HHS/United States
- R01 AI030663-15/AI/NIAID NIH HHS/United States
- R01 AI030663/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials