N-linked glycosylation of west nile virus envelope proteins influences particle assembly and infectivity - PubMed (original) (raw)
N-linked glycosylation of west nile virus envelope proteins influences particle assembly and infectivity
Sheri L Hanna et al. J Virol. 2005 Nov.
Abstract
West Nile virus (WNV) encodes two envelope proteins, premembrane (prM) and envelope (E). While the prM protein of all WNV strains contains a single N-linked glycosylation site, not all strains contain an N-linked site in the E protein. The presence of N-linked glycosylation on flavivirus E proteins has been linked to virus production, pH sensitivity, and neuroinvasiveness. Therefore, we examined the impact of prM and E glycosylation on WNV assembly and infectivity. Similar to other flaviviruses, expression of WNV prM and E resulted in the release of subviral particles (SVPs). Removing the prM glycosylation site in a lineage I or II strain decreased SVP release, as did removal of the glycosylation site in a lineage I E protein. Addition of the E protein glycosylation site in a lineage II strain that lacked this site increased SVP production. Similar results were obtained in the context of either reporter virus particles (RVPs) or infectious lineage II WNV. RVPs or virions bearing combinations of glycosylated and nonglycosylated forms of prM and E could infect mammalian, avian, and mosquito cells (BHK-21, QT6, and C6/36, respectively). Those particles lacking glycosylation on the E protein were modestly more infectious per genome copy on BHK-21 and QT6 cells, while this absence greatly enhanced the infection of C6/36 cells. Thus, glycosylation of WNV prM and E proteins can affect the efficiency of virus release and infection in a manner that is cell type and perhaps species dependent. This suggests a multifaceted role for envelope N-linked glycosylation in WNV biology and tropism.
Figures
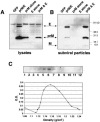
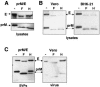
FIG. 1.
Coexpression of viral envelope proteins prM and E together produce SVPs. HEK-293T cells were transfected with plasmids encoding a lineage I prM and E polyprotein (strain NY99-6480) or each envelope protein individually, as indicated. Supernatants and cell lysates were collected 48 h posttransfection and SVPs isolated by ultracentrifugation of supernatant through a 20% sucrose cushion. Both lysates and pelleted SVPs were analyzed by Western blotting using antibodies that recognized the two WNV membrane proteins (αprM/αE). (A) Shown is a representative Western blot of lysates from cells transfected with an irrelevant protein (GFP), the envelope polyprotein (prM/E), prM protein alone, E protein alone, and prM and E expressed from different plasmids. (B) Western blot of the accompanying pelleted SVPs released in the supernatant from each transfection. (C) SVPs produced by transfection of HEK-293T cells with WNV prM/E were pelleted and then layered on the top of a 20 to 60% sucrose gradient and centrifuged for 17 h to allow particles to reach equilibrium. Fractions of equal volume were collected and the refractive index measured for each. Shown is a representative Western blot of fractions collected and probed with a WNV E-specific antibody. Additionally, a plastic capture ELISA was used to measure the WNV antigen quantity in each fraction. Refractive index numbers were converted to density values (g/cm3) and plotted against optical density (O.D.) readings in the ELISA to identify the density at which the majority of particles migrated in the total sample.
FIG. 2.
Glycosylation status of WNV envelope proteins in lysates and released particles. Lysates from HEK-293T cells transfected with a prM/E plasmid (A) or Vero and BHK-21 cells infected with virus (B) were collected 48 h after transfection or infection and treated with glycosidases PNGase F (F), Endo H (H), or buffer only (−) as a control. (C) SVPs pelleted from the HEK-293T cells transfected as shown in panel A or virus produced by the infected Vero cells shown in part B were similarly digested. Shown are Western blots of treated lysates (A and B), SVPs, or virus (C) probed with antibodies that recognize WNV membrane proteins (αprM/αE).
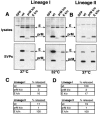
FIG. 3.
Glycosylation influences SVP production. HEK-293T cells were transfected with polyprotein plasmids encoding prM and E from lineage I or lineage II WNV wild type (wt), glycosylation-mutated prM or E, or an irrelevant protein (GFP) as a control. Mutants used were: prM knockout (k/o), which lacked the glycosylation site in prM; E k/o, which lacked the glycosylation site in the E protein of lineage I WNV; and E k/in, which contained a 4-amino-acid insert (NYST) reestablishing the N-linked glycosylation site in the lineage II E protein. Cell lysates and released SVPs were collected 48 h posttransfection. (A) Western blot of lineage I lysates and SVPs from a standard transfection at 37°C probed with antibodies that recognize the WNV membrane proteins (αprM/αE). Repeating the experiment at 32°C did not alter the phenotype of either mutant. (B) Western blot of lineage II lysates and SVPs probed with WNV HIAF. (C) SVP quantified using a WNV E antigen capture ELISA. Data represent three separate experiments normalized by setting the sample from each lineage with the highest release of antigen to 100% release. (D) SVP release was also quantified by metabolically labeling cells expressing the indicated constructs with [35S]methionine-cysteine and measuring the density of the resulting WNV E bands using a phosphorimager.
FIG. 4.
Glycosylation affects reporter virus particle release. (A) Schematic of the three plasmids used to produce RVPs: replicon plasmid (pWIIrep-GFP) encoding the WNV nonstructural proteins (NS1 through NS5), GFP in place of the three structural proteins (C, prM, and E), protease cleavage site (FMDV 2a) and small fragments of C and E needed for proper transcription/translation; an envelope polyprotein (prM/E) plasmid; and capsid plasmid [pWcap(s)]. (B) All three plasmids were transfected into HEK-293T cells, and RVP-containing supernatant was collected 48 h posttransfection. Wild-type (wt) and glycosylation mutant envelopes from both lineage I (NY99-6480) and II (956 D117 3B) strains were used to produce RVPs. A comparison between the measurement of particle release based on antigen content (E antigen capture ELISA) or genome copies (qPCR) from the same RVP preparation is given. For comparison purposes, the results from each assay were normalized by setting the construct from each lineage with the highest production of particles, i.e., the E-glycosylated form in each lineage, to 100% particle release. Values presented are the combined results from three separate assays. Error bars indicate 1 standard deviation from the mean. k/o, knockout; k/in, knockin.
FIG. 5.
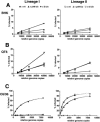
RVP infectivity over multiple dilutions of inocula. RVP infections were normalized based on WNV genome copies per ml in each particle preparation and used to infect target cells over multiple relative genome copies ranging from 40,000 to 750 depending on the cell type. Each infection was carried out in duplicate and cells collected for FACS analysis 40 h p.i. for BHK (A) and QT6 (B) and 72 h p.i. for C6/36 cells (C). Data were plotted according to virus lineage and cell type. The x axis represents the relative genome copies. Percent infection is shown on the y axis and represents the number of GFP-expressing cells as measured by FACS analysis. wt, wild type; k/o, knockout; k/in, knockin.
FIG. 6.
Virus infectivity over multiple dilutions of inocula. Lineage II virus infections (strain 956 D117 3B) were normalized based on WNV genome copies per ml in each viral stock and used to infect target cells over multiple relative genome copies ranging from 50,000 to 750 depending on the cell type. Each infection was carried out in duplicate and cells collected for FACS analysis 12 h p.i. for BHK (A) and QT6 (B) and 24 h p.i. for C6/36 cells (C). The x axis shows the relative genome copies. Percent infection is shown on the y axis and represents the number of GFP-expressing cells as measured by flow cytometry analysis. Points outside the linear range for each infection curve were excluded from the presented data.
Similar articles
- Identification of residues in West Nile virus pre-membrane protein that influence viral particle secretion and virulence.
Setoh YX, Prow NA, Hobson-Peters J, Lobigs M, Young PR, Khromykh AA, Hall RA. Setoh YX, et al. J Gen Virol. 2012 Sep;93(Pt 9):1965-1975. doi: 10.1099/vir.0.044453-0. Epub 2012 Jul 4. J Gen Virol. 2012. PMID: 22764317 - West Nile virus envelope protein glycosylation is required for efficient viral transmission by Culex vectors.
Moudy RM, Zhang B, Shi PY, Kramer LD. Moudy RM, et al. Virology. 2009 Apr 25;387(1):222-8. doi: 10.1016/j.virol.2009.01.038. Epub 2009 Feb 27. Virology. 2009. PMID: 19249803 Free PMC article. - N-linked glycosylation of the West Nile virus envelope protein is not a requisite for avian virulence or vector competence.
Maharaj PD, Langevin SA, Bolling BG, Andrade CC, Engle XA, Ramey WN, Bosco-Lauth A, Bowen RA, Sanders TA, Huang CY, Reisen WK, Brault AC. Maharaj PD, et al. PLoS Negl Trop Dis. 2019 Jul 15;13(7):e0007473. doi: 10.1371/journal.pntd.0007473. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31306420 Free PMC article. - Increased pathogenicity of West Nile virus (WNV) by glycosylation of envelope protein and seroprevalence of WNV in wild birds in Far Eastern Russia.
Kariwa H, Murata R, Totani M, Yoshii K, Takashima I. Kariwa H, et al. Int J Environ Res Public Health. 2013 Dec 12;10(12):7144-64. doi: 10.3390/ijerph10127144. Int J Environ Res Public Health. 2013. PMID: 24351738 Free PMC article. Review. - Post-translational regulation and modifications of flavivirus structural proteins.
Roby JA, Setoh YX, Hall RA, Khromykh AA. Roby JA, et al. J Gen Virol. 2015 Jul;96(Pt 7):1551-69. doi: 10.1099/vir.0.000097. Epub 2015 Feb 23. J Gen Virol. 2015. PMID: 25711963 Review.
Cited by
- Tetraspanins as Potential Therapeutic Candidates for Targeting Flaviviruses.
Ahmed W, Neelakanta G, Sultana H. Ahmed W, et al. Front Immunol. 2021 Apr 21;12:630571. doi: 10.3389/fimmu.2021.630571. eCollection 2021. Front Immunol. 2021. PMID: 33968023 Free PMC article. Review. - N-linked glycosylation attenuates H3N2 influenza viruses.
Vigerust DJ, Ulett KB, Boyd KL, Madsen J, Hawgood S, McCullers JA. Vigerust DJ, et al. J Virol. 2007 Aug;81(16):8593-600. doi: 10.1128/JVI.00769-07. Epub 2007 Jun 6. J Virol. 2007. PMID: 17553891 Free PMC article. - Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence.
Keller BC, Fredericksen BL, Samuel MA, Mock RE, Mason PW, Diamond MS, Gale M Jr. Keller BC, et al. J Virol. 2006 Oct;80(19):9424-34. doi: 10.1128/JVI.00768-06. J Virol. 2006. PMID: 16973548 Free PMC article. - Glycosylation of viral proteins: Implication in virus-host interaction and virulence.
Feng T, Zhang J, Chen Z, Pan W, Chen Z, Yan Y, Dai J. Feng T, et al. Virulence. 2022 Dec;13(1):670-683. doi: 10.1080/21505594.2022.2060464. Virulence. 2022. PMID: 35436420 Free PMC article. Review.
References
- Adams, S. C., A. K. Broom, L. M. Sammels, A. C. Hartnett, M. J. Howard, R. J. Coelen, J. S. Mackenzie, and R. A. Hall. 1995. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 206:49-56. - PubMed
- Beasley, D. W., C. T. Davis, M. Whiteman, B. Granwehr, R. M. Kinney, and A. D. Barrett. 2004. Molecular determinants of virulence of West Nile virus in North America. Arch. Virol. Suppl. 2004:35-41. - PubMed
- Beasley, D. W., L. Li, M. T. Suderman, and A. D. Barrett. 2001. West Nile virus strains differ in mouse neurovirulence and binding to mouse or human brain membrane receptor preparations. Ann. N. Y. Acad. Sci. 951:332-335. - PubMed
- Beasley, D. W., M. C. Whiteman, S. Zhang, C. Y. Huang, B. S. Schneider, D. R. Smith, G. D. Gromowski, S. Higgs, R. M. Kinney, and A. D. Barrett. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 79:8339-8347. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007324/AI/NIAID NIH HHS/United States
- T32 GM007229/GM/NIGMS NIH HHS/United States
- F31 RR005074/RR/NCRR NIH HHS/United States
- U54 AI57168/AI/NIAID NIH HHS/United States
- T32-AI-07324-13/AI/NIAID NIH HHS/United States
- F31 RR05074/RR/NCRR NIH HHS/United States
- T32-GM-007229/GM/NIGMS NIH HHS/United States
- U54 AI057168/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical