Overlapping intracellular and differential synaptic distributions of dopamine D1 and glutamate N-methyl-D-aspartate receptors in rat nucleus accumbens - PubMed (original) (raw)
Overlapping intracellular and differential synaptic distributions of dopamine D1 and glutamate N-methyl-D-aspartate receptors in rat nucleus accumbens
Yuko Hara et al. J Comp Neurol. 2005.
Abstract
The dopamine D1 receptor (D1R) in the nucleus accumbens (Acb) shell is highly implicated in psychostimulant-evoked locomotor activity and reward, whereas the D1R in the Acb core is more crucial for appetitive instrumental learning. These behavioral effects depend in part on interactions involving glutamatergic N-methyl-D-aspartate (NMDA) receptors, whose essential NR1 subunit has physical associations with the D1R. To determine the relevant sites for D1R activation and interactions involving NMDA receptors, we examined the electron microscopic immunolabeling of D1R and NR1 C-terminal peptides in rat Acb shell and core. In each Acb subdivision, the D1Rs were located principally on extrasynaptic plasma membranes of dendritic shafts and spines and more rarely were associated with cytoplasmic endomembranes. Many D1R-labeled somata and dendrites also contained NR1 immunoreactivity. In comparison with D1R, NR1 immunoreactivity was more often seen in the cytoplasm and near asymmetric synapses on somatodendritic profiles. In these profiles, notable overlapping distributions of D1R and NR1 occurred near endomembranes. The exclusively D1R- or D1R- and NR1-containing dendrites were most prevalent in the Acb shell, but were also present in the Acb core. In each region, NR1 was also detected in axon terminals without D1R, which formed excitatory-type synapses with D1R-labeled dendrites. These results provide ultrastructural evidence that D1Rs in the Acb have subcellular distributions supporting, 1) intracellular cotrafficking with NR1 and 2) modulation of the postsynaptic excitability in spiny neurons affected by presynaptic NMDA receptor activation. The region-specific differences in receptor distributions suggest a major, but not exclusive, involvement of Acb D1R in reward-related processing.
Copyright 2005 Wiley-Liss, Inc.
Figures
Figure 1
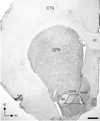
Light micrograph showing immunoperoxidase labeling of D1R in the caudate-putamen nucleus (CPN) and the region of the Acb shell and core that was sampled for electron microscopy. In this half coronal section through the rat brain, the Acb D1R immunoperoxidase labeling is unevenly distributed and especially dense in the shell subregion. Ultrathin sections for electron microscopy were collected from the Acb shell and core directly medial to the anterior commissure (ac) at level 1.6mm anterior to Bregma (Paxinos and Watson, 1986). Each section included both core and shell as indicated by the trapezoid. The myelinated axons in the anterior commissure and the base of the trapezoid were used for orientation in the analysis of electron microscopic images. cc, corpus callosum; CTX, cortex; lv, lateral ventricle. Corner arrows indicate the dorsal (d) and medial (m) brain regions. Scale bar, 500 µm.
Figure 2
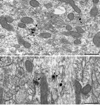
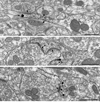
Electron micrographs from Acb shell showing somatodendritic co-localization of D1R and NR1. A. In a soma, D1R immunogold particles (arrowheads) are present near the Golgi (G) and endomembranes (em), where there is partial overlap with diffuse NR1 immunoperoxidase product (arrow). B. Within a dually labeled large proximal dendrite (D1&NR1-De), one D1R immunogold particle (arrowhead) superimposes NR1 immunoperoxidase labeling (arrow) near membranous organelles in the central portion of the cytoplasm. Scale bar, 0.5 µm.
Figure 3
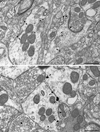
Plasmalemmal D1R immunogold labeling in dendritic profiles within the Acb shell. A. D1R immunogold particles (arrowheads) in a dendrite (D1-De) and spine (D1-Sp) are located at the plasma membrane apposed by unlabeled small unmyelinated axons (ua1&2) and axon terminals (ut1&2). The spine receives an asymmetric synapse (curved arrow) from an unlabeled terminal (ut3). B. D1R immunogold particles (arrowheads) in a dendrite (D1-De1) are located on the plasma membrane apposed by another D1R-labeled dendrite (D1-De2). Both dendrites are apposed by a single unlabeled terminal (ut), which shows a symmetric synapse (dotted arrow) exclusively with D1-De1. Scale bar, 0.5 µm.
Figure 4
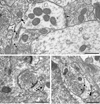
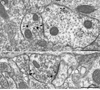
D1R immunogold labeling in dendritic spines in Acb shell (A, B) and core (C). A. D1R gold particles (arrowheads) are located along the plasma membrane in the neck of a dendritic spine (D1-Sp) apposed by unlabeled axons (ua1&2) and a small glial process (asterisk). The dendrite also shows an obliquely sectioned synapse from an unlabeled terminal (ut) that appears to be asymmetric (curved arrow). B. D1R gold particles (arrowheads) are seen on or near the extrasynaptic plasmalemma of a dendritic spine (D1-Sp). The immunogold particles are apposed by a glial process (asterisk). The spine receives input from an unlabeled terminal (ut) making an asymmetric synapse (curved arrow). C. One D1R gold particle (arrowhead) is located on the perisynaptic membrane of a dendritic spine (D1-Sp), while the others are on the extrasynaptic membrane. The gold particles are apposed by an unlabeled axon (ua) and a glial process (asterisk). The spine receives an asymmetric synapse (curved arrow) from an unlabeled terminal (ut). sa, spine apparatus. Scale bar, 0.5 µm.
Figure 5
D1R and NR1 distributions in common spines in Acb shell. A. In a dually labeled spine (D1&NR1-Sp), D1R immunoperoxidase reactivity (straight arrow) is most prominent on the plasmalemma of a process resembling the neck of a spine, originating from a dendrite (De) without detectable labeling. NR1 immunogold aggregate (arrowhead) is located in more distal portions of the spinous process near an asymmetric synapse (curved arrow) from an unlabeled terminal (ut). B. NR1 immunogold particles (arrowheads) are located in the cytoplasm within and near the postsynaptic membrane, while D1R immunoperoxidase products are seen diffusely throughout a dually-labeled dendritic spine (D1&NR1-Sp). This spine is receiving a perforated excitatory-type synapse (curved arrow). C. D1R immunogold particles (arrowheads) in a dually-labeled dendritic spine (D1&NR1-Sp) are located within the cytoplasm and on extrasynaptic portions of the plasmalemma apposing an unlabeled axononal profile (ua). NR1 immunoperoxidase reaction products are diffusely distributed throughout the cytoplasm and along the dendritic specialization (straight arrow) of an asymmetric synapse (curved arrow). The presynaptic terminal is unlabeled and contains a mixture of small clear and large dense-core vesicles (dcv). us, unlabeled spine. Scale bar, 0.5 µm.
Figure 6
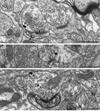
D1R immunogold labeling in axon terminals in Acb shell (A) and core (B). A. Two immunogold particles (arrowheads) are located in the central portions of an axon terminal (D1-Te). This terminal is joined by a symmetric synapse (dotted arrow) to a dendrite containing one D1R immunogold particle in the cytoplasm near an endomembranous structure (em) presumed to be smooth ER. B. D1R immunogold particles (arrowheads) are located on the extrasynaptic plasma membrane of an axon terminal (D1-Te), near appositions with two unlabeled axon terminals (ut1, ut2). Another particle in the same terminal is in the cytosol near the presynaptic specialization at an asymmetric synapse (curved arrow). us, unlabeled spine. scv, small clear vesicle. Scale bar, 0.5 µm.
Figure 7
D1R immunoperoxidase labeled dendritic spines (D1-Sp) receiving asymmetric synapses (curved arrow) from NR1 immunogold-labeled axon terminals (NR1-Te) in the Acb shell (A) and core (B, C). A. NR1 immunogold particles (arrowheads) are located on the extrasynaptic plasma membrane of an axon terminal (NR1-Te). The D1R immunoperoxidase products are prevalent along the extrasynaptic plasmalemma (arrow), but also diffusely distributed within the D1-Sp. B. An NR1 immunogold particle (arrowhead) is located on the extrasynaptic plasma membrane of a small axon terminal (NR1-Te). C. Two NR1 immunogold particles (arrowheads) are distributed on the extrasynaptic membrane of an axon terminal (NR1-Te) and another is located in the cytosol near small clear vesicles. us, unlabeled spine. ua, unlabeled axon. Scale bar, 0.5 µm.
Figure 8
Bar graphs showing the number and types of neuronal profiles immunolabeled for D1R, D1R and NR1, or NR1 in the rat Acb medial shell and core. Data were obtained from one vibratome section from each of three rats. For each rat, an area of at least 3400 µm2 of neuropil was examined in each subregion, and the labeled profiles were identified and counted. Both D1R and NR1 labeling are observed in somatodendritic and axonal profiles. ANOVA shows that there are significantly more D1R-labeled dendrites in the shell than in the core (*p<0.01). Also, significantly more dendrites are dual-labeled in the Acb shell than in the core (*p<0.01). No other profiles showed significant shell and core differences in number.
Figure 9
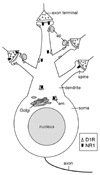
Schematic diagram showing the subcellular distributions of D1R (white isosceles triangles) and NR1 (black chevrons) in a medium spiny Acb neuron. In the soma and proximal dendrite, D1R and NR1 are located in close proximity to each other near endomembranes (em). In a small-medium dendrite, D1R is present on the plasma membrane and NR1 is present in the cytoplasm. A dendritic spine containing both D1R and NR1 is shown on the top. In this spine, NR1 is located near the excitatory-type asymmetric synapse, while D1R is present on the extrasynaptic plasma membrane apposed by an axonal profile (ap). A D1R dendritic spine receiving an excitatory input from an NR1-containing terminal is shown on the left. A dendritic spine expressing D1R alone is shown on the top right. In this spine, D1R is located on the extrasynaptic plasma membrane, apposed by an axonal profile. A dendritic spine containing NR1 near the excitatory synapse is shown on the bottom right. This drawing does not indicate that all of these spines originate from the same neuron.
Similar articles
- Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens.
Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM. Glass MJ, et al. Exp Neurol. 2008 Apr;210(2):750-61. doi: 10.1016/j.expneurol.2008.01.012. Epub 2008 Jan 26. Exp Neurol. 2008. PMID: 18294632 Free PMC article. - Dopamine D1 receptors have subcellular distributions conducive to interactions with prodynorphin in the rat nucleus accumbens shell.
Hara Y, Yakovleva T, Bakalkin G, Pickel VM. Hara Y, et al. Synapse. 2006 Jul;60(1):1-19. doi: 10.1002/syn.20273. Synapse. 2006. PMID: 16575853 - Dendritic distributions of dopamine D1 receptors in the rat nucleus accumbens are synergistically affected by startle-evoking auditory stimulation and apomorphine.
Hara Y, Pickel VM. Hara Y, et al. Neuroscience. 2007 Jun 8;146(4):1593-605. doi: 10.1016/j.neuroscience.2007.04.005. Epub 2007 May 9. Neuroscience. 2007. PMID: 17490822 Free PMC article. - Deletion of the NMDA-NR1 receptor subunit gene in the mouse nucleus accumbens attenuates apomorphine-induced dopamine D1 receptor trafficking and acoustic startle behavior.
Glass MJ, Robinson DC, Waters E, Pickel VM. Glass MJ, et al. Synapse. 2013 Jun;67(6):265-79. doi: 10.1002/syn.21637. Epub 2013 Mar 5. Synapse. 2013. PMID: 23345061 Free PMC article.
Cited by
- MicroRNA expression profile and functional analysis reveal that miR-382 is a critical novel gene of alcohol addiction.
Li J, Li J, Liu X, Qin S, Guan Y, Liu Y, Cheng Y, Chen X, Li W, Wang S, Xiong M, Kuzhikandathil EV, Ye JH, Zhang C. Li J, et al. EMBO Mol Med. 2013 Sep;5(9):1402-14. doi: 10.1002/emmm.201201900. Epub 2013 Jul 22. EMBO Mol Med. 2013. PMID: 23873704 Free PMC article. - Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens.
Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM. Glass MJ, et al. Exp Neurol. 2008 Apr;210(2):750-61. doi: 10.1016/j.expneurol.2008.01.012. Epub 2008 Jan 26. Exp Neurol. 2008. PMID: 18294632 Free PMC article. - Dissociable control of motivation and reinforcement by distinct ventral striatal dopamine receptors.
Enriquez-Traba J, Arenivar M, Yarur-Castillo HE, Noh C, Flores RJ, Weil T, Roy S, Usdin TB, LaGamma CT, Wang H, Tsai VS, Kerspern D, Moritz AE, Sibley DR, Lutas A, Moratalla R, Freyberg Z, Tejeda HA. Enriquez-Traba J, et al. Nat Neurosci. 2025 Jan;28(1):105-121. doi: 10.1038/s41593-024-01819-9. Epub 2024 Dec 9. Nat Neurosci. 2025. PMID: 39653808 Free PMC article. - Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice.
Gan JO, Bowline E, Lourenco FS, Pickel VM. Gan JO, et al. Neuroscience. 2014 Jan 31;258:174-83. doi: 10.1016/j.neuroscience.2013.11.003. Epub 2013 Nov 11. Neuroscience. 2014. PMID: 24231734 Free PMC article. - Appetitive cue-evoked ERK signaling in the nucleus accumbens requires NMDA and D1 dopamine receptor activation and regulates CREB phosphorylation.
Kirschmann EK, Mauna JC, Willis CM, Foster RL, Chipman AM, Thiels E. Kirschmann EK, et al. Learn Mem. 2014 Oct 16;21(11):606-15. doi: 10.1101/lm.035113.114. Print 2014 Nov. Learn Mem. 2014. PMID: 25322796 Free PMC article.
References
- Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. N-methyl-D-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–59. - PubMed
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. - PubMed
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem. 2002;77:44–62. - PubMed
- Brady AE, Limbird LE. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal. 2002;14:297–309. - PubMed
- Breukel AI, Besselsen E, Lopes da Silva FH, Ghijsen WE. A presynaptic N-methyl-D-aspartate autoreceptor in rat hippocampus modulating amino acid release from a cytoplasmic pool. Eur J Neurosci. 1998;10:106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH40342/MH/NIMH NIH HHS/United States
- DA04600/DA/NIDA NIH HHS/United States
- R37 MH040342/MH/NIMH NIH HHS/United States
- R01 DA004600/DA/NIDA NIH HHS/United States
- R01 MH040342/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases