A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells - PubMed (original) (raw)
A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells
J A Alvarado et al. Br J Ophthalmol. 2005 Nov.
Abstract
Aim: To test the hypothesis that trabecular meshwork endothelial cells (TMEs) increase the permeability of Schlemm's canal endothelial cells (SCEs) by actively releasing ligands that modulate the barrier properties of SCEs.
Methods: The TMEs were first irradiated with a laser light and allowed to condition the medium, which is then added to SCEs. The treatment response is determined by both measuring SCE permeability (flow meters) and the differential expression of genes (Affymetrix chips and quantitative polymerase chain reaction (PCR)). The cytokines secreted by the treated cells were identified using ELISA and the ability of these cytokines to increase permeability is tested directly after their addition to SCEs in perfusion experiments.
Results: SCEs exposed to medium conditioned by the light activated TMEs (TME-cm) respond by undergoing a differential expression (DE) of 1,120 genes relative to controls. This response is intense relative to a DE of only 12 genes in lasered SCEs. The TME-cm treatment of SCEs increased the SCE permeability fourfold. The role of cytokines in these responses is supported by two findings: adding specific cytokines established to be secreted by lasered TMEs to SCEs increases permeability; and inactivating the TME-cm by boiling or diluting, abrogates these conditioned media permeability effects.
Conclusion: These experiments show that TMEs can regulate SCE permeability and that it is likely that TMEs have a major role in the regulation of aqueous outflow. This novel TME driven cellular mechanism has important implications for the pathogenesis of glaucoma and the mechanism of action of laser trabeculoplasty. Ligands identified as regulating SCE permeability have potential use for glaucoma therapy.
Figures
Figure 1
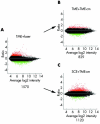
Ratio intensity scatter plot comparing the profile of genes expressed by TMEs treated by the application of short pulse/green light delivered by a frequency doubled Nd:YAG laser instrument (A); by the addition of media conditioned by the irradiated TMEs (TME-cm) when added to naive TMEs (B); or by adding TME-cm to naive SCEs (C). Each dot represents the mean intensity log ratio (base 2) versus the average log intensity of a single gene based on four replicas (that is, one chip for each of four samples), with each replicate containing 11 probes/gene, using approximately 47 000 transcripts from 38 500 well characterised genes. Red and green dots indicate upregulated and downregulated genes, respectively, demonstrating a twofold or greater differential expression (DE) ratio with the total number of differentially expressed genes (DEGs) for each category indicated below each graph.
Figure 2
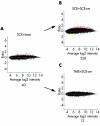
Ratio intensity scatter plot as in figure 1 showing profiles DEGs in SCEs after the lasering procedure (A); or after adding SCE-cm to naive SCEs (B); or after adding SCE-cm to naive TMEs (C).
Figure 3
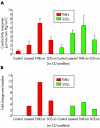
(A) The conductivity responses in μl/min/mm Hg/cm2, with the mean (SD), measured in monolayers of both TMEs and SCEs in controls and in treated preparations as indicated. In (B) the treatment responses are depicted adjusting for differences in baseline as a fold change. Note that in all cases, the responses are robust, amounting to increases of over 11-fold in the case of the TME-cm, the highest response, and 200% in the case of naive SCEs treated with SCE-cm, the smallest response.
Figure 4
Conductivity responses measured in μl/min/mm Hg/cm2 showing the mean and SD in controls (blue) and in TMEs treated by the addition of media conditioned by light irradiated TMEs (red). Adding media from naive TMEs, or from laser activated TMEs that had been inactivated by dilution or boiling effectively abrogated the increase in conductivity (blue), relative to the situation when media conditioned by light irradiated TMEs was added (red).
Figure 5
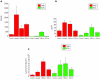
Intensity plot depicting the mRNA responses (means (SD)) measured using quantitative PCR (Q-PCR) in the eight experimental and control preparations (A), and at the protein level measured using ELISA (B). These responses are correlated with those measured in conductivity assays (C). The mRNA responses are measured as the mean intensity log ratio (base 2). The ELISA measurements are expressed in pg/ml, and the conductivity studies units in μl/min/mm Hg/cm2.
Figure 6
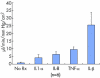
Plot showing changes in conductivity induced by the direct addition to SCE monolayers of 10 ng of IL1-α, 5 ng of IL-8, 15 ng of TNF-α, and 10 ng of IL-β?. Mean (SD) are shown.
Similar articles
- Interactions between endothelia of the trabecular meshwork and of Schlemm's canal: a new insight into the regulation of aqueous outflow in the eye.
Alvarado JA, Yeh RF, Franse-Carman L, Marcellino G, Brownstein MJ. Alvarado JA, et al. Trans Am Ophthalmol Soc. 2005;103:148-62; discussion 162-3. Trans Am Ophthalmol Soc. 2005. PMID: 17057799 Free PMC article. - Profiling of Cytokines Secreted by Conventional Aqueous Outflow Pathway Endothelial Cells Activated In Vitro and Ex Vivo With Laser Irradiation.
Alvarado JA, Chau P, Wu J, Juster R, Shifera AS, Geske M. Alvarado JA, et al. Invest Ophthalmol Vis Sci. 2015 Nov;56(12):7100-8. doi: 10.1167/iovs.15-17660. Invest Ophthalmol Vis Sci. 2015. PMID: 26529044 Free PMC article. - From the bedside to the bench and back again: predicting and improving the outcomes of SLT glaucoma therapy.
Alvarado JA, Iguchi R, Juster R, Chen JA, Shifera AS. Alvarado JA, et al. Trans Am Ophthalmol Soc. 2009 Dec;107:167-81. Trans Am Ophthalmol Soc. 2009. PMID: 20126493 Free PMC article. - [Aspects of aqueous humor drainage through Schlemm's canal].
Bill A, Mäepea O, Hamanaka T. Bill A, et al. Klin Monbl Augenheilkd. 1989 Nov;195(5):277-80. doi: 10.1055/s-2008-1050038. Klin Monbl Augenheilkd. 1989. PMID: 2689763 Review. German. - How does the trabecular meshwork regulate outflow? Clues from the vascular endothelium.
Brandt JD, O'Donnell ME. Brandt JD, et al. J Glaucoma. 1999 Oct;8(5):328-39. J Glaucoma. 1999. PMID: 10529934 Review.
Cited by
- Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine.
Fini ME, Schwartz SG, Gao X, Jeong S, Patel N, Itakura T, Price MO, Price FW Jr, Varma R, Stamer WD. Fini ME, et al. Prog Retin Eye Res. 2017 Jan;56:58-83. doi: 10.1016/j.preteyeres.2016.09.003. Epub 2016 Sep 22. Prog Retin Eye Res. 2017. PMID: 27666015 Free PMC article. Review. - Human stem cells home to and repair laser-damaged trabecular meshwork in a mouse model.
Yun H, Wang Y, Zhou Y, Wang K, Sun M, Stolz DB, Xia X, Ethier CR, Du Y. Yun H, et al. Commun Biol. 2018 Dec 6;1:216. doi: 10.1038/s42003-018-0227-z. eCollection 2018. Commun Biol. 2018. PMID: 30534608 Free PMC article. - Focus on molecular events in the anterior chamber leading to glaucoma.
Saccà SC, Izzotti A. Saccà SC, et al. Cell Mol Life Sci. 2014 Jun;71(12):2197-218. doi: 10.1007/s00018-013-1493-z. Epub 2013 Oct 19. Cell Mol Life Sci. 2014. PMID: 24142347 Free PMC article. Review. - Goniodysgenesis variability and activity of CYP1B1 genotypes in primary congenital glaucoma.
García-Antón MT, Salazar JJ, de Hoz R, Rojas B, Ramírez AI, Triviño A, Aroca-Aguilar JD, García-Feijoo J, Escribano J, Ramírez JM. García-Antón MT, et al. PLoS One. 2017 Apr 27;12(4):e0176386. doi: 10.1371/journal.pone.0176386. eCollection 2017. PLoS One. 2017. PMID: 28448622 Free PMC article.
References
- Hogan M, Alvarado J, Weddell J. Histology of the human eye: an atlas and textbook. Philadelphia: WB Saunders, 1971:1–687.
- Alvarado J, Betanzos A, Franse-Carman L, et al. Endothelia of schlemm’s canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am J Physiol Cell Physiol 2004;286:C621–34. - PubMed
- Alvarado J, Franse-Carman L, McHolm G, et al. The response of the meshwork cells to adrenergic agents and their antagonists. In: Krieglstein, ed. Glaucoma update IV. Heidelberg: Springer Verlag, 1991:9–19.
- Alvarado JA, Murphy CM, Franse-Carman L, et al. Effect of beta-adrenergics on paracellular width and fluid flow across outflow pathway cells. Invest Ophthalmol Vis Sci 1998;39:1813–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources