gamma-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide-binding site - PubMed (original) (raw)
gamma-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide-binding site
Patrick C Fraering et al. J Biol Chem. 2005.
Abstract
gamma-Secretase is an unusual protease with an intramembrane catalytic site that cleaves many type I membrane proteins, including the amyloid beta-protein (Abeta) precursor (APP) and the Notch receptor. Genetic and biochemical studies have identified four membrane proteins as components of gamma-secretase: heterodimeric presenilin composed of its N- and C-terminal fragments, nicastrin, Aph-1, and Pen-2. Here we demonstrated that certain compounds, including protein kinase inhibitors and their derivatives, act directly on purified gamma-secretase to selectively block cleavage of APP- but not Notch-based substrates. Moreover, ATP activated the generation of the APP intracellular domain and Abeta, but not the generation of the Notch intracellular domain by the purified protease complex, and was a direct competitor of the APP-selective inhibitors, as were other nucleotides. In accord, purified gamma-secretase bound specifically to an ATP-linked resin. Finally, a photoactivable ATP analog specifically labeled presenilin 1-C-terminal fragments in purified gamma-secretase preparations; the labeling was blocked by ATP itself and APP-selective gamma-secretase inhibitors. We concluded that a nucleotide-binding site exists within gamma-secretase, and certain compounds that bind to this site can specifically modulate the generation of Abeta while sparing Notch. Drugs targeting the gamma-secretase nucleotide-binding site represent an attractive strategy for safely treating Alzheimer disease.
Figures
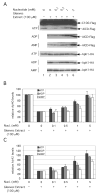
FIGURE 1. Nonhydrolyzed ATP can activate the in vitro generation of Aβ40 and Aβ42
A, effect of ATP on purified γ-secretase activity. γ-Secretase diluted in 0.2% CHAPSO/HEPES, pH 7.5, was incubated at 37 °C for 4 h in the presence of 0.1% PC, 0.025% PE, the indicated concentrations of ATP, and 1 μ
m
C100FLAG (an APP-based substrate) or 1 μ
m
N100FLAG (a Notch-based substrate); both substrates were adjusted to 0.5% SDS prior to addition to the reactions (6). The reactions were Western-blotted for AICD-FLAG (with M2 anti-FLAG antibody) and for NICD-FLAG (with Notch Ab1744 antibody). Levels of Aph1-HA serve as equal loading controls. Aβ40 and Aβ42 were measured by ELISA (B, means ± S.D.; n = 3). Levels of AICD-FLAG and NICD-FLAG were estimated by densitometry (values are single determinations from the blot shown). Asterisks indicate significant differences in Aβ40 (*, p < 0.01) and Aβ42 (**, p < 0.01) production compared with samples without ATP. C, ATPase assays on purified γ-secretase. [α-32P]ATP was incubated at 37 °C for the indicated times in the reaction buffer (0.2% CHAPSO/HEPES, pH 7.5, 150 m
m
NaCl, 5 m
m
MgCl2, 5 m
m
CaCl2, 0.025% PE, and 0.10% PC) alone (lanes 11–15) or in the presence of purified γ-secretase (lanes 6 –10), C100FLAG substrate (lanes 16 –20), or both purified γ-secretase and C100FLAG substrate (lanes 21–25). Two μl of each reaction were then analyzed by TLC to separate ATP from ADP. As a positive control to show ATP hydrolysis products, [α-32P]ATP was incubated at the indicated times in the presence of canine kidney phosphatase (lanes 1–5).
FIGURE 2. Gleevec itself is not a direct γ-secretase inhibitor, but a Gleevec extract inhibits the generation of Aβ by purified γ-secretase without affecting the cleavage of a Notch-based substrate
A, effect of III-31C and DAPT on the cleavage by purified γ-secretase of C100FLAG and N100FLAG. γ-Secretase diluted in 0.2% CHAPSO/HEPES, pH 7.5, was incubated at 37 °C for 4 h with 1 μ
m
C100FLAG or N100FLAG substrate, 0.1% PC, 0.025% PE, and the indicated concentrations of III-31C and DAPT. The reactions were Western-blotted for AICD-FLAG (M2 antibody) and NICD-FLAG (Ab1744 antibody). Levels of Aph1-HA serve as equal loading controls. B, effect of Gleevec extract on Aβ40 and Aβ42 generation by purified γ-secretase. γ-Secretase diluted in 0.2% CHAPSO/HEPES, pH 7.5, was incubated at 37 °C for 4 h with 1 μ
m
C100FLAG substrate, 0.1% PC, 0.025% PE, and the indicated concentrations of Gleevec extract. Aβ40 and Aβ42 were measured by ELISA (means ± S.D., n = 3). C, effects of Gleevec extract on C100FLAG and N100FLAG cleavage by purified γ-secretase. Parallel reaction mixtures in the presence of 1 μ
m
C100FLAG or N100FLAG, 10 μ
m
III-31C, or the indicated concentrations of the Gleevec extract were incubated at 37 °C for 4 h and Western-blotted for AICD-FLAG (M2), Aβ (6E10), and for NICD-FLAG (Ab1744); short exposures (short exp.) are also shown to validate the lack of effect on NICD-FLAG levels. Levels of Aph1-HA serve as equal loading controls. D, effect of Gleevec extract on C100FLAG and N100FLAG cleavage by endogenous γ-secretase solubilized from HeLa membranes. γ-Secretase assays and the generation of AICD-FLAG, Aβ, and NICD-FLAG were performed as in C. Levels of NCT serve as loading controls. E, effect of three different Gleevec samples on C100FLAG cleavage by purified γ-secretase. γ-Secretase was incubated as described in B with C100FLAG and the indicated concentrations of Gleevec extracted from capsules (left panel), Gleevec extracted from tablets (middle panel), or purified Gleevec (right panel). The reactions were Western-blotted for AICD-FLAG and Aβ. Levels of Aph1-HA serve as equal loading controls. Final purity and characterization of the three Gleevec samples was performed by MALDI-TOF mass spectroscopy; the compounds identified specifically in the active Gleevec extract (left panel) are labeled with arrowheads. Note that a very minor peak at 286.6 in the inactive extract is a major peak in the active extract (asterisk).
FIGURE 3. Nucleotides prevent the inhibitory effect of Gleevec extract on purified γ-secretase
A, ATP, ADP, and AMP are direct competitors with respect to the Gleevec extract. γ-Secretase diluted in 0.2% CHAPSO/HEPES, pH 7.5, was incubated at 37 °C for 16 h in the absence (lane 1) or presence (lanes 2– 6) of 100 μ
m
Gleevec extract and the indicated concentrations of ATP, ADP, or AMP. The generation of AICD-FLAG was probed by Western blotting with M2 anti-FLAG. Levels of Aph1-HA served as equal loading controls. Note that the lane 1 control without ATP is the same control for the absence of ADP or AMP. B and C, the effects of increasing concentrations of ATP, ADP, and AMP on Aβ40 (B) and Aβ42 (C) generation by purified γ-secretase in the presence of 100 μ
m
Gleevec extract (reactions performed at 37 °C for 4 h) were quantified by ELISA (n = 3).
FIGURE 4. ZM39923 (1367), a potent Janus kinase 3 inhibitor, preferentially blocks the generation of Aβ by purified γ-secretase
A, effect of a large number of protein kinase/phosphatase inhibitors or activators on purified γ-secretase activity. γ-Secretase diluted in 0.2% CHAPSO/HEPES, pH 7.5, was incubated at 37 °C for 16 h in the presence of 1 μ
m
C100FLAG, 0.1% PC, 0.025% PE, and 100 μ
m
of the indicated compounds, except for III-31C (10 μ
m
). The generation of AICD-FLAG was probed by Western blotting with M2 anti-FLAG antibody. B, effect of ZM39923 (1367) and its break-down product ZM449829 (1366) (structures shown) on the cleavage by purified γ-secretase of C100FLAG and N100FLAG. Activity assays were performed as described above in the presence of the indicated concentrations of 1367 and 1366, and the generation of AICD-FLAG and NICD-FLAG was probed by Western blotting with M2 and Notch Ab1744 antibody, respectively. In all figures, levels of Aph1-HA are shown as equal loading controls. C, similarly, the effect of SC-9 (0433) (structure shown) on the cleavage by purified γ-secretase of C100FLAG and N100FLAG was probed.
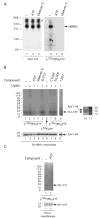
FIGURE 5. Purified γ-secretase binds specifically to an ATP resin
Purified γ-secretase (St. indicates starting material) was incubated overnight in the presence or absence of 50 m
m
ATP with two different ATP-immobilized resins as follows: ATP attached to acrylamide through the γ-phosphate (lanes 1–9) or ATP attached to agarose through the ribose hydroxyls (lanes 10 –12). The unbound fractions (Unb.) were recovered, and the resins washed three times in 0.2% CHAPSO/HEPES and resuspended in Laemmli sample buffer to recover the bound proteins (P. indicates precipitate). All samples were electrophoresed on 4 –20% Tris-glycine gels and transferred to polyvinylidene difluoride membranes to detect NCT-GST (αGST), PS1-NTF (Ab14), Aph1-HA (3F10), PS1-CTF (13A1), and FLAG-Pen2 (M2-anti FLAG). Note that purified γ-secretase binds specifically to the resin in which ATP is attached to acrylamide through the γ-phosphate (lanes 1– 6), whereas γ-secretase from a crude lysate (of γ-30 cells) is unable to bind to the same resin (lanes 7–9). Starting material (St.) and unbound (Unb.) lanes were each loaded with the equivalent of 25% of the material that was bound to the resins (P.) so that the unbound and bound protein levels can be compared directly.
FIGURE 6. Specific labeling of the γ-secretase component PS1-CTF with 8-azido-[γ-32P]ATP, a photoaffinity ATP analog
A, photolabeling of γ-secretase with 8-azido-[γ-32P]ATP as revealed by nondenaturing BN-PAGE. Purified γ-secretase solubilized in 0.1% digitonin/Tris-buffered saline was incubated with 22.5 μ
m
of 8-azido-[γ-32P]ATP (10 μCi per reaction) in the presence of 10 m
m
ATP (lanes 2 and
) or 1 mm Gleevec extract (Gleevec E., lanes 3 and ), exposed to UV light for 5 min, and subjected to BN-PAGE analysis as described (31). 32P labeling was assessed by autoradiography (lanes 4 – 6) (BioMax MS films used with BioMax Transcreen HE; Kodak). As a control for the migration of γ-secretase, the purified complex solubilized in 0.1% digitonin/Tris-buffered saline (lane 1), in the presence of 10 mm ATP (lane 2) or 1 mm Gleevec extract (lane 3), was exposed to UV light for 5 min, subjected to BN-PAGE analysis on the same gel as above, and probed for the γ-secretase complex using 3F10 antibody to the Aph1-HA component (lanes 1–3). The asterisk denotes nonspecific aggregates containing Aph1-HA (P. C. Fraering, W. Ye, M. J. LaVoie, B. L. Ostaszewski, D. J. Selkoe, and M. S. Wolfe, unpublished data). HMWC, the high molecular weight γ-secretase complex (31). B, photolabeling of γ-secretase with 8-azido-[γ-32P]ATP as revealed by denaturing SDS-PAGE. Purified γ-secretase was incubated with 22.5 μm of 8-azido-[γ-32P]ATP (10 μCi per reaction) in the absence (lane 1) or presence (lane 2) of PC and PE (lipids) or in the presence of 10 mm ATP (lane 3), 1 mm Gleevec extract (lane 4), 1 μm III-31C (lane 5), 1 μm C100FLAG (lane 7), 1 μm N100FLAG (lane 8), or 300 μm 1367 (lane 9). As a control for the specificity of photolabeling, purified γ-secretase was also incubated with [γ-32P]ATP (without the 8-azido group) (lane 6). All samples were exposed to UV light for 5 min, and the reactions were quenched with 1 mm dithiothreitol. The samples were diluted and were incubated overnight at 4 °C with GSH resin for the affinity precipitation of the NCT-GST component and its associated proteins (6). The resins were then washed three times, and all precipitated proteins were electrophoresed on 4 –20% Tris-glycine gels and the photolabeled proteins autoradiographed as in A. As a control for the mobility of the photolabeled proteins, the same sample as shown in lane 1 was electrophoresed on a 4 –20% Tris-glycine gel and Western-blotted simultaneously for PS1-CTF and Aph1-HA with 13A11 and 3F10 antibodies, respectively (lane 10). Molecular weight markers are in lane 11. The levels of the Aph1-HA on the gel in B are shown to demonstrate equal protein loading. C, photolabeling of endogenous γ-secretase with 8-azido-[γ-32P]ATP. Membranes from untransfected HeLa cells were incubated with 8-azido-[γ-32P]ATP (10 μCi per reaction) in the absence (lane 1) or presence (lane 2) of 10 mm ATP and exposed to UV light as described above. The membranes were washed; the proteins were solubilized in 1% CHAPSO/HEPES, and γ-secretase was co-immunoprecipitated with anti-PS1-NTF antibodies. The photolabeled proteins were detected as described above, and levels of PS1-NTF (Western-blotted with mAb1563 antibody) serve as co-immunoprecipitated controls.
Similar articles
- Purification and characterization of the human gamma-secretase complex.
Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Fraering PC, et al. Biochemistry. 2004 Aug 3;43(30):9774-89. doi: 10.1021/bi0494976. Biochemistry. 2004. PMID: 15274632 - Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s).
Kimberly WT, Esler WP, Ye W, Ostaszewski BL, Gao J, Diehl T, Selkoe DJ, Wolfe MS. Kimberly WT, et al. Biochemistry. 2003 Jan 14;42(1):137-44. doi: 10.1021/bi026888g. Biochemistry. 2003. PMID: 12515548 - Gamma-secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage.
Chyung JH, Raper DM, Selkoe DJ. Chyung JH, et al. J Biol Chem. 2005 Feb 11;280(6):4383-92. doi: 10.1074/jbc.M409272200. Epub 2004 Nov 29. J Biol Chem. 2005. PMID: 15569674 - Inhibition of gamma-secretase as a therapeutic intervention for Alzheimer's disease: prospects, limitations and strategies.
Evin G, Sernee MF, Masters CL. Evin G, et al. CNS Drugs. 2006;20(5):351-72. doi: 10.2165/00023210-200620050-00002. CNS Drugs. 2006. PMID: 16696577 Review. - Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex.
De Strooper B. De Strooper B. Neuron. 2003 Apr 10;38(1):9-12. doi: 10.1016/s0896-6273(03)00205-8. Neuron. 2003. PMID: 12691659 Review.
Cited by
- Possible mechanisms of action of NSAIDs and related compounds that modulate gamma-secretase cleavage.
Kukar T, Golde TE. Kukar T, et al. Curr Top Med Chem. 2008;8(1):47-53. doi: 10.2174/156802608783334042. Curr Top Med Chem. 2008. PMID: 18220932 Free PMC article. - Presenilins and γ-secretase: structure, function, and role in Alzheimer Disease.
De Strooper B, Iwatsubo T, Wolfe MS. De Strooper B, et al. Cold Spring Harb Perspect Med. 2012 Jan;2(1):a006304. doi: 10.1101/cshperspect.a006304. Cold Spring Harb Perspect Med. 2012. PMID: 22315713 Free PMC article. Review. - 100 years and counting: prospects for defeating Alzheimer's disease.
Roberson ED, Mucke L. Roberson ED, et al. Science. 2006 Nov 3;314(5800):781-4. doi: 10.1126/science.1132813. Science. 2006. PMID: 17082448 Free PMC article. Review. - BACE and gamma-secretase characterization and their sorting as therapeutic targets to reduce amyloidogenesis.
Marks N, Berg MJ. Marks N, et al. Neurochem Res. 2010 Feb;35(2):181-210. doi: 10.1007/s11064-009-0054-1. Epub 2009 Sep 17. Neurochem Res. 2010. PMID: 19760173 Review. - Part 1: Notch-sparing γ-secretase inhibitors: The identification of novel naphthyl and benzofuranyl amide analogs.
Lu D, Wei HX, Zhang J, Gu Y, Osenkowski P, Ye W, Selkoe DJ, Wolfe MS, Augelli-Szafran CE. Lu D, et al. Bioorg Med Chem Lett. 2016 May 1;26(9):2129-32. doi: 10.1016/j.bmcl.2016.03.040. Epub 2016 Mar 12. Bioorg Med Chem Lett. 2016. PMID: 27013392 Free PMC article.
References
- Selkoe DJ. Physiol Rev. 2001;81:741–766. - PubMed
- Vassar R, Citron M. Neuron. 2000;27:419–422. - PubMed
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Nature. 1999;398:513–517. - PubMed
- Kopan R, Ilagan MX. Nat Rev Mol Cell Biol. 2004;5:499–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG015379/AG/NIA NIH HHS/United States
- AG15379/AG/NIA NIH HHS/United States
- NS41355/NS/NINDS NIH HHS/United States
- R01 AG017574/AG/NIA NIH HHS/United States
- AG17574/AG/NIA NIH HHS/United States
- R01 NS041355/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources