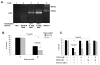Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans - PubMed (original) (raw)
. 2005 Dec 30;280(52):42655-42668.
doi: 10.1074/jbc.M505910200. Epub 2005 Oct 19.
Shamol Saha # 1, Beth Westlund 2, Celine Perier 3, Lucinda Burnam 2, Anne Sluder 2, Marius Hoener 4, Cecilia Mp Rodrigues 5, Aixa Alfonso 6, Clifford Steer 7, Leo Liu 2, Serge Przedborski 3, Benjamin Wolozin 1
Affiliations
- PMID: 16239214
- PMCID: PMC3910375
- DOI: 10.1074/jbc.M505910200
Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans
Rina Ved et al. J Biol Chem. 2005.
Abstract
How genetic and environmental factors interact in Parkinson disease is poorly understood. We have now compared the patterns of vulnerability and rescue of Caenorhabditis elegans with genetic modifications of three different genetic factors implicated in Parkinson disease (PD). We observed that expressing alpha-synuclein, deleting parkin (K08E3.7), or knocking down DJ-1 (B0432.2) or parkin produces similar patterns of pharmacological vulnerability and rescue. C. elegans lines with these genetic changes were more vulnerable than nontransgenic nematodes to mitochondrial complex I inhibitors, including rotenone, fenperoximate, pyridaben, or stigmatellin. In contrast, the genetic manipulations did not increase sensitivity to paraquat, sodium azide, divalent metal ions (Fe(II) or Cu(II)), or etoposide compared with the nontransgenic nematodes. Each of the PD-related lines was also partially rescued by the antioxidant probucol, the mitochondrial complex II activator, D-beta-hydroxybutyrate, or the anti-apoptotic bile acid tauroursodeoxycholic acid. Complete protection in all lines was achieved by combining d-beta-hydroxybutyrate with tauroursodeoxycholic acid but not with probucol. These results show that diverse PD-related genetic modifications disrupt the mitochondrial function in C. elegans, and they raise the possibility that mitochondrial disruption is a pathway shared in common by many types of familial PD.
Figures
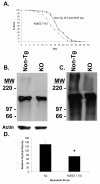
Figure 1. Verification of α-synuclein expression and knockout of K08E3.7 in transgenic C. elegans strains
(A) PCR analysis of expressed wild type and A53T α-synuclein transgenes. 450 bp band is present in the wild type (WT) and A53T α-synuclein expressing lines (lanes 1 and 2) and co-migrates with a band amplified from α-synuclein cDNA (lane 4). The 450 bp α-synuclein-specific band is absent from the non-tg lane (lane 3). (B) Immunoprecipitation of α-synuclein demonstrates the presence of the α-synuclein protein (lanes 2 and 4, left panel). No synuclein is present in lysates from non-tg worms (lanes 1 and 3, left panel) nor in lysates immunoprecipitated with non-specific IgG (right panel). The doublet pattern of reactivity is often seen in immunoblots of α-synuclein. (C) PCR of genomic DNA across the KO8E3.7 gene yields a 1.37 Kb band for non-tg nematodes (lane 1) and a 0.26 Kb band for the K08E3.7 KO strain (lane 2). (D) The amino acid sequence alignment of K08E3.7 and human parkin. Exact amino acid matches are indicated with the identical amino acid in between the K08E3.7 and human parkin sequences; conservative amino acid differences are identified by a ‘+’. The N-terminal ubiquitin-like domain (shaded box – upper right), RING finger domains (boxed), in-between RING domain (shaded box) are indicated. (E) Diagram of the full-length K08E3.7 gene and the K08E3.7 gene containing the 1132 bp deletion from the K08E3.7 knockout.
Figure 2. Characterization of the K08E3.7 KO transgenic strain
(A) A life span curve shows that K08E3.7 KO (square) strain has life span that is 15.4% shorter than the non-tg (diamond), wild type (WT, triangle) and A53T (circle) α-synuclein expressing strains. Mean life span for the non-tg, WT and A53T α-synuclein expressing strains was 18.5 ± 0.169, while the mean life span of the K08E3.7 KO strain was 15.65 ± 0.211. p<0.01 compared to the non-tg. (B) Immunoblot probed with monoclonal ubiquitin antibody (upper panel) or actin (lower panel, loading control) showing higher levels of high molecular weight ubiquitin conjugates present in the non-tg (lane 1) strain than the K08E3.7 knockout (KO, lane 2) strain. (C) Longer exposure of immunoblot shows high molecular weight smears of ubiquitin conjugates in the stacking gel in the lysates from the non-tg strain. (D) Densitometric quantification of immunoblot from panel B blot; (*) p<0.01.
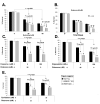
Figure 3. K08E3.7 KO, wild type and A53T α-synuclein expressing lines are selectively vulnerable to rotenone-induced toxicity
(A & B.) K08E3.7 KO, wild type and A53T α-synuclein expressing lines show increased vulnerability to rotenone at varying doses (A; 25 or 50 μM rotenone, 4 days treatment), and at different times (B; 50 μM rotenone, 2 and 4 days treatment). The PD-related lines show enhanced vulnerability after 4 days of rotenone treatment; (*) p<0.01. At 50 μM rotenone, the A53T α-synuclein expressing strain is also more vulnerable than the wild type α-synuclein strain; (#) p<0.01. (+) p <0.01 compared to untreated of the same strain. (C – E) The K08E3.7 KO, wild type and A53T α-synuclein expressing strains show enhanced toxicity compared to the non-tg line after treatment for 4 days with complex I inhibitors fenperoximate (C), stigmatellin (D) and pyridaben (E), (*) p<0.01, (+) p<0.01 compared to untreated nematodes of the same line.
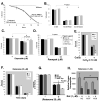
Figure 4. Vulnerability to other toxins unchanged in K08E3.7 KO, wild type and A53T α-synuclein expressing lines
(A.) Kill curve over a 24-hour treatment with 500mM sodium azide. Neither the K08E3.7 KO, wild type nor A53T α-synuclein expressing strains show increased sensitivity to sodium azide compared to the non-tg strain; (+) p<0.01. (B. & C.) No difference in sensitivity to iron (II) (B), copper (II) (B), or paraquat (C) compared to the non-tg strain. (D) Both wild type and A53T α-synuclein are slightly protective against etoposide treatment, while K08E3.7 KO strain is slightly vulnerable compared to the non-tg strain. (E) The P301L tau C. elegans line shows increased vulnerability to copper compared to non-tg or BY200 worm lines after 6 days of exposure to 0.75 mM CuCl2. (F) The P301L tau C. elegans line shows no difference in vulnerability to rotenone (25 μM) compared to non-tg or BY200 worm lines (*) p<0.01 compared to non-tg. (G) The β-synuclein expressing C. elegans line shows no difference in vulnerability to rotenone (25 μM) compared to non-tg, while the A53T α-synuclein line shows increased toxicity (*) p<0.001 compared to non-tg & β-syn, N=8. (H) The K08E3.7 KO and A53T α-synuclein show reduced oxygen consumption under basal conditions compared to the non-tg line. (+) p <0.01 compared to untreated nematodes of the same strain.
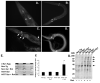
Figure 5. Increased fibrillogenisis in the A53T α-synuclein expressing line
(A) GFP fluorescence present in dopaminergic neurons of BY200 worms after treatment with 25 μM rotenone for 2 days. (B) GFP fluorescence present in dopaminergic neurons of A53T α-synuclein worms after treatment with 25 μM rotenone for 2 days. (C & D) Staining with thioflavine S highlights additional fluorescence (arrows) present in A53T α-synuclein worms after treatment with 25 μM rotenone for 2 days, suggesting the presence of protein aggregates. (E) Slot blot analysis of non-tg and A53Tα-synculein expressing strain lysates using the Syn303 antibody that recognizes fibrillar α-synuclein. Row 1 – Recombinant aggregated α-synuclein (aged 1 month); Row 2 – non-tg strain; Row 3 – non-tg treated with rotenone (25 μM, 4 days); Row 4 – A53T α-synuclein expressing strain; Row 5 – A53T α-synuclein expressing strain treated with rotenone (25 μM, 4 days). Lysates were analyzed in triplicate. Aggregated α-synuclein was observed in the recombinant α-synuclein and in the A53T α-synuclein expressing strain treated with rotenone. (F) Quantification of slot blot analysis; (*) p<0.01 compared to non-tg. (G) Oxyblot analysis of total lysates from C. elegans treated with 25 μM rotenone for 2 days shows increased protein oxidation (arrows).
Figure 6. Protection against rotenone-induced toxicity
(A) Co-treatment of the K08E3.7 KO and A53T α-synuclein expressing strains with rotenone and probucol yielded partial protection of the non-tg, K08E3.7 KO and A53T α-synuclein strains compared to untreated of the same strain; (+) p<0.01. (B & C) Co-treatment of the non-tg, K08E3.7 KO and A53T α-synuclein expressing strains with rotenone and DβHB (panel B) or TUDCA (panel C) fully protected the non-tg strain, but partially protected the K08E3.7 KO and A53T α-synuclein expressing strains compared to untreated nematodes of the same strain (+) p<0.01. Treatment with probucol, DβHB or TUDCA was unable to completely overcome the enhanced rotenone-induced toxicity of the transgenic strains as compared to the non-tg strain in any treatment group; (*) p<0.01. (D) Both probucol and DβHB provided partial protection against rotenone for K08E3.7 KO and A53T α-synuclein expressing strains, but did not show any additive benefit when combined together; (+) p<0.01 comparing the treatments, and (*) p<0.01 comparing the strains. (E) Probucol also did not show any additive benefit against rotenone toxicity when combined with TUDCA; (+) p<0.01 comparing the treatments, and (*) p<0.01 comparing the strains. (F) Combining DβHB and TUDCA fully protected against rotenone-induced toxicity in all the strains compared to untreated of the same strain; (+) p<0.01.
Figure 7. Analysis of the mechanisms of action of TUDCA and paraquat
(A) Treatment with TUDCA does not protect against rotenone-induced toxicity in the Ced-3 knockout worm compared to the untreated group; (+) p<0.01. The Ced-3 knockout worms was less vulnerable to rotenone toxicity than the non-tg strain, but addition of TUDCA did not provide any additional protection; (*) p<0.01. (B) Treatment with DβHB did not protect the non-tg, K08E3.7 KO or A53T α-synuclein expressing strain against paraquat induced toxicity. (+) p<0.01 compared to untreated of the same strain. (C) Treatment with probucol partially protects against paraquat induced toxicity in the non-tg, K08E3.7 KO and A53T α-synuclein expressing strains; (#) p<0.01 compared to paraquat treated of the same strain. (+) p<0.01 compared to untreated of the same strain.
Figure 8. Knockdown of DJ-1 increases vulnerability to rotenone and is rescued by DβHB and TUDCA
(A) Knockdown of DJ-1 increased the vulnerability to rotenone-induced toxicity. Lane 1: H2O alone, Lane 2: RNA from the bacteria containing the B0432.2 (DJ-1) fragment used for the knockdown, Lane 3: RNA from C. elegans used for B0432.2 (DJ-1) knockdown, Lane 4: RNA from C. elegans used for Sel-9 knockdown, Lane 5: DNA standards. Lane 2 did not produce an amplification product because the procedure for isolating RNA effectively removed the DNA plasmid used to generate the RNAi. (B) Knockdown of DJ-1 increased the vulnerability to rotenone-induced toxicity. (C) Treatment with DβHB and TUDCA fully protected both the non-tg strain and the DJ-1 knockdown nematodes; (+) p<0.01 compared to untreated nematodes of the same line. (*) p<0.01 as compared to non-tg of the same treatment group.
Similar articles
- Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1.
Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Kamp F, et al. EMBO J. 2010 Oct 20;29(20):3571-89. doi: 10.1038/emboj.2010.223. Epub 2010 Sep 14. EMBO J. 2010. PMID: 20842103 Free PMC article. - Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.
Büeler H. Büeler H. Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review. - Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation.
Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z. Xiong H, et al. J Clin Invest. 2009 Mar;119(3):650-60. doi: 10.1172/JCI37617. Epub 2009 Feb 23. J Clin Invest. 2009. PMID: 19229105 Free PMC article. - α-synuclein expression from a single copy transgene increases sensitivity to stress and accelerates neuronal loss in genetic models of Parkinson's disease.
Cooper JF, Spielbauer KK, Senchuk MM, Nadarajan S, Colaiácovo MP, Van Raamsdonk JM. Cooper JF, et al. Exp Neurol. 2018 Dec;310:58-69. doi: 10.1016/j.expneurol.2018.09.001. Epub 2018 Sep 5. Exp Neurol. 2018. PMID: 30194957 Free PMC article. - [The role of parkin in Parkinson's disease].
Miklya I, Göltl P, Hafenscher F, Pencz N. Miklya I, et al. Neuropsychopharmacol Hung. 2014 Jun;16(2):67-76. Neuropsychopharmacol Hung. 2014. PMID: 24978049 Review. Hungarian.
Cited by
- Mitochondrial dynamics: the intersection of form and function.
Ferree A, Shirihai O. Ferree A, et al. Adv Exp Med Biol. 2012;748:13-40. doi: 10.1007/978-1-4614-3573-0_2. Adv Exp Med Biol. 2012. PMID: 22729853 Free PMC article. Review. - Antipurinergic therapy corrects the autism-like features in the Fragile X (Fmr1 knockout) mouse model.
Naviaux JC, Wang L, Li K, Bright AT, Alaynick WA, Williams KR, Powell SB, Naviaux RK. Naviaux JC, et al. Mol Autism. 2015 Jan 13;6:1. doi: 10.1186/2040-2392-6-1. eCollection 2015. Mol Autism. 2015. PMID: 25705365 Free PMC article. - The divalent metal transporter homologues SMF-1/2 mediate dopamine neuron sensitivity in caenorhabditis elegans models of manganism and parkinson disease.
Settivari R, Levora J, Nass R. Settivari R, et al. J Biol Chem. 2009 Dec 18;284(51):35758-68. doi: 10.1074/jbc.M109.051409. J Biol Chem. 2009. PMID: 19801673 Free PMC article. - Using Caenorhabditis elegans to Model Therapeutic Interventions of Neurodegenerative Diseases Targeting Microbe-Host Interactions.
Wang C, Zheng C. Wang C, et al. Front Pharmacol. 2022 Apr 28;13:875349. doi: 10.3389/fphar.2022.875349. eCollection 2022. Front Pharmacol. 2022. PMID: 35571084 Free PMC article. Review. - Tauroursodeoxycholic acid: a potential therapeutic tool in neurodegenerative diseases.
Khalaf K, Tornese P, Cocco A, Albanese A. Khalaf K, et al. Transl Neurodegener. 2022 Jun 4;11(1):33. doi: 10.1186/s40035-022-00307-z. Transl Neurodegener. 2022. PMID: 35659112 Free PMC article. Review.
References
- Dauer W, Przedborski S. Neuron. 2003;39(6):889–909. - PubMed
- Langston J. Ann. Neurol. 1998;44(Suppl. 1):S45–52. - PubMed
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Nat Neurosci. 2000;3(12):1301–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG/NS17485/AG/NIA NIH HHS/United States
- R01 NS041786/NS/NINDS NIH HHS/United States
- R01 NS060872/NS/NINDS NIH HHS/United States
- R01 ES015567/ES/NIEHS NIH HHS/United States
- NS41786/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous