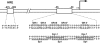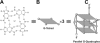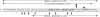Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents - PubMed (original) (raw)
Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents
Daekyu Sun et al. Nucleic Acids Res. 2005.
Abstract
The proximal promoter region of the human vascular endothelial growth factor (VEGF) gene contains a polypurine/polypyrimidine tract that serves as a multiple binding site for Sp1 and Egr-1 transcription factors. This tract contains a guanine-rich sequence consisting of four runs of three or more contiguous guanines separated by one or more bases, corresponding to a general motif for the formation of an intramolecular G-quadruplex. In this study, we observed the progressive unwinding of the oligomer duplex DNA containing this region into single-stranded forms in the presence of KCl and the G-quadruplex-interactive agents TMPyP4 and telomestatin, suggesting the dynamic nature of this tract under conditions which favor the formation of the G-quadruplex structures. Subsequent footprinting studies with DNase I and S1 nucleases using a supercoiled plasmid DNA containing the human VEGF promoter region also revealed a long protected region, including the guanine-rich sequences, in the presence of KCl and telomestatin. Significantly, a striking hypersensitivity to both nucleases was observed at the 3'-side residue of the predicted G-quadruplex-forming region in the presence of KCl and telomestatin, indicating altered conformation of the human VEGF proximal promoter region surrounding the guanine-rich sequence. In contrast, when specific point mutations were introduced into specific guanine residues within the G-quadruplex-forming region (Sp1 binding sites) to abolish G-quadruplex-forming ability, the reactivity of both nucleases toward the mutated human VEGF proximal promoter region was almost identical, even in the presence of telomestatin with KCl. This comparison of wild-type and mutant sequences strongly suggests that the formation of highly organized secondary structures such as G-quadruplexes within the G-rich region of the human VEGF promoter region is responsible for observed changes in the reactivity of both nucleases within the polypurine/polypyrimidine tract of the human VEGF gene. The formation of the G-quadruplex structures from this G-rich sequence in the human VEGF promoter is further confirmed by the CD experiments. Collectively, our results provide strong evidence that specific G-quadruplex structures can naturally be formed by the G-rich sequence within the polypurine/polypyrimidine tract of the human VEGF promoter region, raising the possibility that the transcriptional control of the VEGF gene can be modulated by G-quadruplex-interactive agents.
Figures
Figure 1
Polypurine/polypyrimidine sequence located upstream (−89 to −43) of the promoter region of the VEGF gene. Runs of guanines (GR-I through GR-V) are boxed. Binding sites of the transcriptional factors Egr-1 and Sp1 are underlined.
Figure 2
(A) H-bonding pattern in a G-tetrad, (B) schematic diagram of the G-tetrad and (C) cartoon of an 18mer parallel G-quadruplex representing that found in the c-MYC promoter region.
Figure 3
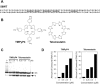
The effect of increasing TMPyP4 and telomestatin concentrations on the conversion of 59mer duplex DNA (59WT) to alternative secondary structures. (A) Nucleotide sequence of 59WT. (B) Structures of TMPyP4 and telomestatin. (C) Autoradiogram of DNA breathing assay. Duplex DNA was titrated with TMPyP4 or telomestatin in 100 mM KCl/TE buffer at 37 or 42°C. Two bands corresponding to Watson–Crick duplex (DS) and single-stranded DNA (S) were identified, along with an isomeric form. (D) Graphical representation of data described in (C).
Figure 4
In vitro footprinting of the VEGF promoter region with DNase I. (A) Autoradiograms showing DNase I cleavage sites on the top strand of a supercoiled pGL3-VEGF plasmid. The plasmid DNA was incubated in the absence of salt (lane 1), or in the presence of 100 mM KCl without (lane 2) and with (lane 3) 1 µM telomestatin at 37°C for 1 h before digesting with DNase I. DNase I cleavage sites were mapped using linear amplification by PCR with 32P-labeled gene-specific plasmid DNA pretreated with DNase I. Arrows A and B indicate the hypersensitive cleavage sites to nucleases. (B) Densitometric scanning of the autoradiogram in (A). The bars indicate the guanine repeats involved in the formation of the G-quadruplex structures. Arrows A and B indicate the hypersensitive cleavage sites to nucleases. (C) Autoradiograms showing DNase I cleavage sites on the bottom strand of a supercoiled pGL3-VEGF plasmid. The designation of lanes 1–3 was as in (A) above. DNase I cleavage sites were mapped using linear amplification by PCR with 32P-labeled gene-specific plasmid DNA pretreated with DNase I. The vertical bar next to the gel indicates the polypyrimidine tract.
Figure 5
In vitro footprinting of the VEGF promoter region with S1 nuclease. (A) Autoradiograms showing S1 nuclease cleavage sites on the top strand of a supercoiled pGL3-VEGF plasmid. Arrow A indicates the hypersensitive cleavage sites to S1 nuclease. (B) Densitometric scanning of the autoradiogram in (A). The plasmid DNA was incubated in the absence of salt (lane 1) or in the presence of 100 mM KCl without (lane 2) and with (lane 3) 1 µM telomestatin at 37°C for 1 h before digesting with S1 nuclease. S1 nuclease cleavage sites were mapped using linear amplification by PCR with 32P-labeled gene-specific primers on plasmid DNA pretreated with S1 nuclease. Arrow A indicates the hypersensitive cleavage sites to S1 nuclease. (C) Autoradiograms showing S1 cleavage sites on the bottom strand of a supercoiled pGL3-VEGF plasmid. The designation of lanes 1–3 was as in (A) above. The vertical bar next to the gel indicates the polypyrimidine tract and the arrows indicate the S1 nuclease hypersensitivity sites.
Figure 6
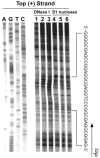
DNA polymerase stop assay to determine the ability of the VEGF promoter to form G-quadruplex structures. (A and B) The wild-type (WT) and mutant (MT) template sequences (shown below the gels) with increasing concentrations of K+ (0–100 mM). Arrows indicate the positions of the full-length product (F) of DNA synthesis, the G-quadruplex pause site (S), and the free primer (P). Lanes A, G, T and C represent dideoxy sequencing reactions with the same template as a size marker for the precise arrest sites.
Figure 7
In vitro footprinting of the mutant VEGF promoter region with DNase I and S1 nuclease. Autoradiograms showing DNase I (lanes 1–3) and S1 (lanes 4–6) cleavage sites on the top strand of a supercoiled pGL3-VEGFM17 plasmid. This plasmid was incubated in the absence of salt (lanes 1 and 4) or in the presence of 100 mM KCl without (lanes 2 and 5) and with (lanes 3 and 6) 1 µM telomestatin at 37°C for 1 h before digesting with nucleases. Nuclease cleavage sites were mapped using linear amplification by PCR with 32P-labeled gene-specific primers on mutant plasmid DNA pretreated with S1 nuclease or DNase I.
Figure 8
CD spectra of the VEGF-Pu20T, d(T5G3CG3C2G5CG3T5), in Tris–HCl buffer (20 mM, pH 7.6) in the presence of increasing concentrations of KCl (0, 10, 50 and 100 mM). Each spectrum corresponds to four averaged scans taken at 25°C and is baseline corrected for signal contributions due to the buffer.
Figure 9
Summary of the results from both DNase I and S1 nuclease footprinting (Figures 4 and 5). The arrow heads and filled circles indicate the hypersensitive sites to S1 nuclease and DNase I, respectively.
Similar articles
- The proximal promoter region of the human vascular endothelial growth factor gene has a G-quadruplex structure that can be targeted by G-quadruplex-interactive agents.
Sun D, Liu WJ, Guo K, Rusche JJ, Ebbinghaus S, Gokhale V, Hurley LH. Sun D, et al. Mol Cancer Ther. 2008 Apr;7(4):880-9. doi: 10.1158/1535-7163.MCT-07-2119. Mol Cancer Ther. 2008. PMID: 18413801 Free PMC article. - Formation of pseudosymmetrical G-quadruplex and i-motif structures in the proximal promoter region of the RET oncogene.
Guo K, Pourpak A, Beetz-Rogers K, Gokhale V, Sun D, Hurley LH. Guo K, et al. J Am Chem Soc. 2007 Aug 22;129(33):10220-8. doi: 10.1021/ja072185g. Epub 2007 Aug 2. J Am Chem Soc. 2007. PMID: 17672459 Free PMC article. - Evidence of the formation of G-quadruplex structures in the promoter region of the human vascular endothelial growth factor gene.
Sun D, Guo K, Shin YJ. Sun D, et al. Nucleic Acids Res. 2011 Mar;39(4):1256-65. doi: 10.1093/nar/gkq926. Epub 2010 Oct 18. Nucleic Acids Res. 2011. PMID: 20959293 Free PMC article. - G-quadruplexes as targets for drug design.
Hurley LH, Wheelhouse RT, Sun D, Kerwin SM, Salazar M, Fedoroff OY, Han FX, Han H, Izbicka E, Von Hoff DD. Hurley LH, et al. Pharmacol Ther. 2000 Mar;85(3):141-58. doi: 10.1016/s0163-7258(99)00068-6. Pharmacol Ther. 2000. PMID: 10739869 Review. - Structures, folding patterns, and functions of intramolecular DNA G-quadruplexes found in eukaryotic promoter regions.
Qin Y, Hurley LH. Qin Y, et al. Biochimie. 2008 Aug;90(8):1149-71. doi: 10.1016/j.biochi.2008.02.020. Epub 2008 Feb 29. Biochimie. 2008. PMID: 18355457 Free PMC article. Review.
Cited by
- Targeting DNA G-quadruplex structures with peptide nucleic acids.
Panyutin IG, Onyshchenko MI, Englund EA, Appella DH, Neumann RD. Panyutin IG, et al. Curr Pharm Des. 2012;18(14):1984-91. doi: 10.2174/138161212799958440. Curr Pharm Des. 2012. PMID: 22376112 Free PMC article. Review. - DNA G-Quadruplex in Human Telomeres and Oncogene Promoters: Structures, Functions, and Small Molecule Targeting.
Chen L, Dickerhoff J, Sakai S, Yang D. Chen L, et al. Acc Chem Res. 2022 Sep 20;55(18):2628-2646. doi: 10.1021/acs.accounts.2c00337. Epub 2022 Sep 2. Acc Chem Res. 2022. PMID: 36054116 Free PMC article. - Solution-state structure of an intramolecular G-quadruplex with propeller, diagonal and edgewise loops.
Marusic M, Sket P, Bauer L, Viglasky V, Plavec J. Marusic M, et al. Nucleic Acids Res. 2012 Aug;40(14):6946-56. doi: 10.1093/nar/gks329. Epub 2012 Apr 24. Nucleic Acids Res. 2012. PMID: 22532609 Free PMC article. - Recognition and Unfolding of c-MYC and Telomeric G-Quadruplex DNAs by the RecQ C-Terminal Domain of Human Bloom Syndrome Helicase.
Lee S, Kim J, Han S, Park CJ. Lee S, et al. ACS Omega. 2020 Jun 11;5(24):14513-14522. doi: 10.1021/acsomega.0c01176. eCollection 2020 Jun 23. ACS Omega. 2020. PMID: 32596589 Free PMC article. - Dual Targeting Topoisomerase/G-Quadruplex Agents in Cancer Therapy-An Overview.
Salerno S, Barresi E, Baglini E, Poggetti V, Taliani S, Da Settimo F. Salerno S, et al. Biomedicines. 2022 Nov 15;10(11):2932. doi: 10.3390/biomedicines10112932. Biomedicines. 2022. PMID: 36428499 Free PMC article. Review.
References
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. - PubMed
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29:15–18. - PubMed
- Giordano F.J., Johnson R.S. Angiogenesis: the role of the microenvironment in flipping the switch. Curr. Opin. Genet. Dev. 2001;11:35–40. - PubMed
- Goodsell D.S. The molecular perspective: VEGF and angiogenesis. Stem Cells. 2003;21:118–119. - PubMed
- Jain R.K. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin. Oncol. 2002;29:3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources