Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes - PubMed (original) (raw)
. 2005 Nov;115(11):3256-64.
doi: 10.1172/JCI25105. Epub 2005 Oct 20.
Affiliations
- PMID: 16239964
- PMCID: PMC1257537
- DOI: 10.1172/JCI25105
Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes
Marloes A Naarding et al. J Clin Invest. 2005 Nov.
Abstract
DC-specific ICAM3-grabbing non-integrin (DC-SIGN), which is expressed on DCs, can interact with a variety of pathogens such as HIV-1, hepatitis C, Ebola, cytomegalovirus, Dengue virus, Mycobacterium, Leishmania, and Candida albicans. We demonstrate that human milk can inhibit the DC-SIGN-mediated transfer of HIV-1 to CD4+ T lymphocytes as well as viral transfer by both immature and mature DCs. The inhibitory factor directly interacted with DC-SIGN and prevented the HIV-1 gp120 envelope protein from binding to the receptor. The human milk proteins lactoferrin, alpha-lactalbumin, lysozyme, beta-casein, and secretory leukocyte protease inhibitor did not bind DC-SIGN or demonstrate inhibition of viral transfer. The inhibitory effect could be fully alleviated with an Ab recognizing the Lewis X (LeX) sugar epitope, commonly found in human milk. LeX in polymeric form or conjugated to protein could mimic the inhibitory activity, whereas free LeX sugar epitopes could not. We reveal that a LeX motif present in human milk can bind to DC-SIGN and thereby prevent the capture and subsequent transfer of HIV-1 to CD4+ T lymphocytes. The presence of such a DC-SIGN-binding molecule in human milk may both influence antigenic presentation and interfere with pathogen transfer in breastfed infants.
Figures
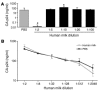
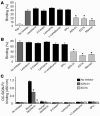
Figure 1
Direct infection of CD4+ T lymphocytes in the presence of human milk. (A) PBS or several dilutions of human milk from an uninfected mother were spiked with LAI (X4) and added to CD4-enriched T lymphocytes. After a 2-hour incubation, the CD4+ T lymphocytes were washed, and fresh medium was added. (B) LAI (X4) was incubated with a 1:2 dilution of human milk or PBS for 2 hours, after which several dilutions were made and added to CD4+ T lymphocytes. For both experiments the CA-p24 concentration was determined on day 7. *P < 0.05 compared with the PBS control.
Figure 2
DC-SIGN–dependent transfer of HIV-1 to CD4+ T lymphocytes is inhibited in the presence of human milk. (A) A 1:2 dilution of human milk of an uninfected mother or PBS was spiked with primary isolates NSI-18 (R5) or SI-19 (X4) before addition to Raji-DC-SIGN cells. After an incubation of 30 minutes or 2 hours, the cells were washed, and activated CD4+ T lymphocytes were added. Viral replication was measured on days 7, 9, 12, and 14 after infection by determining CA-p24 values using a standard ELISA. The bars represent maximum and minimum CA-p24 values. (B) PBS or serial dilutions of human milk were spiked with JR-CSF (R5) or LAI (X4) before addition of Raji-DC-SIGN cells; after an incubation of 2 hours, the cells were washed with PBS, and stimulated CD4+ T lymphocytes were added. At day 7, CA-p24 concentrations were determined by standard ELISA. Percent inhibition was determined in reference to the CA-p24 concentration of the corresponding spiked PBS control.
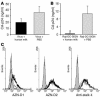
Figure 3
The human milk compound(s) interact with the DC-SIGN receptor, which does not lead to DC-SIGN downmodulation. (A) Human milk (1:4) or PBS was preincubated with a high-titer stock of LAI before adding to Raji-DC-SIGN cells at a dilution known not to inhibit viral replication. After incubation, the cells were washed, and CD4+ T lymphocytes were added, with CA-p24 values measured on day 15 by standard ELISA (P > 0.01). (B) Human milk (1:4) or PBS were incubated with Raji-DC-SIGN, after which the cells were washed to remove unbound human milk components before addition of LAI. After incubation, the cells were washed again, and CD4+ T lymphocytes were added, with the CA-p24 values measured on day 15 by standard ELISA. *P < 0.01. (C) Raji-DC-SIGN cells were incubated with TSM or human milk (1:2) before the binding of AZN-D1, AZN-D2, and anti-stalk 4 DC-SIGN–specific Abs were determined. The filled histograms represent the isotype control; the black lines represent the Ab binding without human milk preincubation; and the dotted lines represent the Ab binding after the cells were incubated with human milk.
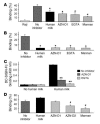
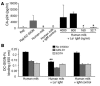
Figure 4
DC-SIGN-Fc binding ELISA and the gp120 bead adhesion assay demonstrate the interaction of the human milk compound(s) with DC-SIGN. (A and B) Raji-DC-SIGN cells or iDCs, respectively, were incubated with human milk (1:20) before addition of fluorescent gp120–coated beads. DC-SIGN–positive cells and mock Raji cells were incubated with buffer as controls. To determine the specificity of the observed binding, the cells were incubated with AZN-D1, EGTA, and mannan before addition of the gp120 beads. *P < 0.05 compared with the PBS control. (C) Human milk (1:20) was coated before addition of DC-SIGN-Fc. The specificity of the observed binding was determined by the preincubation of DC-SIGN-Fc with AZN-D1 and EGTA. **P < 0.01 compared with the noninhibitory control. (D) Raji cells expressing the L-SIGN receptor were incubated with buffer, human milk (1:20), AZN-D1, AZN-D2, or mannan before addition of the gp120 fluorescent beads. #P < 0.01 compared with the binding without an inhibitor.
Figure 5
Human milk inhibits the transfer of HIV-1 by iDCs and mDCs. (A) Both iDCs and mDCs from the same donor were incubated with several dilutions of human milk for 30 minutes before addition of LAI (X4). After 2 hours the cells were washed, and LuSIV cells were added; after 24 hours the LuSIV cells were washed, and the luciferase activity was determined as described in Methods. The asterisks represent statistical differences in infections (P < 0.05). (B) After an incubation of iDCs with human milk or PBS, the cells were washed and LAI was added. After an incubation of 2 hours, the cells were washed again, and captured CA-p24 levels were monitored via ELISA. **P < 0.05 compared with the corresponding control value for both experiments. (C) iDCs were incubated with TSM or human milk (1:2) before the binding of AZN-D1, AZN-D2, and anti-stalk 4 DC-SIGN–specific Abs were determined. The filled histograms represent the isotype control; the black lines represent the Ab binding without human milk preincubation; and the dotted lines represent the Ab binding after the cells were incubated with human milk.
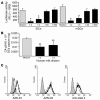
Figure 6
The major milk proteins are not responsible for the inhibitory effect of human milk. (A and B) Raji-DC-SIGN cells or iDCs were incubated with the major milk proteins before addition of fluorescent gp120–coated beads; control cells were incubated with buffer. To determine the specificity of the observed binding, the cells were incubated with AZN-D1, EGTA, and mannan before addition of the gp120 beads. The asterisks represent P < 0.01 compared with noninhibitory control. (C) The major milk proteins were coated on ELISA plates, and DC-SIGN-Fc binding was measured. To determine the specificity of the observed binding, the DC-SIGN-Fc was preincubated with AZN-D1 and EGTA. *P < 0.01 compared with both the AZN-D1 and EGTA control. In all experiments the major proteins were diluted to a 1:20 dilution of their physiological concentration in human milk.
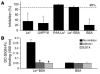
Figure 7
Incubation of human milk (1:20) with LeX IgM Ab relieves the inhibitory properties of human milk on DC-SIGN–mediated transfer of HIV-1 to CD4+ T lymphocytes. (A) A 1:200 dilution of human milk was incubated alone, with IgM control Ab (4,000 ng/ml), or with serial dilutions of LeX IgM Ab (4,000 to 32.7 ng/ml) before addition of Raji-DC-SIGN cells. LAI was added, and following a short incubation, the cells were washed, and activated CD4+ T lymphocytes were added, with CA-p24 values determined at day 7. *P < 0.05 compared with the Raji-DC-SIGN control. (B) Human milk (1:200) was coated and preincubated with anti-LeX IgM Ab (4,000 ng/ml) or an IgM control Ab (4,000 ng/ml) before addition of DC-SIGN-Fc to determine binding. DC-SIGN-Fc was preincubated with AZN-D1and EGTA to determine the specificity of the observed binding. **P < 0.01 compared with the human milk binding without Ab present.
Figure 8
Multimeric and protein-associated LeX inhibits DC-SIGN–mediated viral transfer. (A) Several LeX-containing compounds showed a difference in their ability to block DC-SIGN–dependent transfer of HIV-1 to CD4+ T lymphocytes. The LeX trisaccharide, LNFP III, PAA-LeX, LeX-BSA, and control BSA were tested in the Raji-DC-SIGN culture transfer assay at concentrations of 10 μg/ml. The inhibition is depicted as a percentage of the Raji-DC-SIGN incubated with PBS. (B) LeX-BSA and BSA as a control were coated before addition of DC-SIGN-Fc to determine the binding. DC-SIGN-Fc was preincubated with AZN-D1and EGTA to determine the specificity of the observed binding. *P < 0.01 compared with both the AZN-D1 and EGTA control.
Similar articles
- Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells.
Naarding MA, Dirac AM, Ludwig IS, Speijer D, Lindquist S, Vestman EL, Stax MJ, Geijtenbeek TB, Pollakis G, Hernell O, Paxton WA. Naarding MA, et al. Antimicrob Agents Chemother. 2006 Oct;50(10):3367-74. doi: 10.1128/AAC.00593-06. Antimicrob Agents Chemother. 2006. PMID: 17005819 Free PMC article. - Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes.
Stax MJ, van Montfort T, Sprenger RR, Melchers M, Sanders RW, van Leeuwen E, Repping S, Pollakis G, Speijer D, Paxton WA. Stax MJ, et al. Virology. 2009 Sep 1;391(2):203-11. doi: 10.1016/j.virol.2009.06.011. Virology. 2009. PMID: 19682628 - MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells.
Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, van Kooyk Y. Saeland E, et al. Mol Immunol. 2009 Jul;46(11-12):2309-16. doi: 10.1016/j.molimm.2009.03.025. Epub 2009 Apr 29. Mol Immunol. 2009. PMID: 19406479 - DC-SIGN points the way to a novel mechanism for HIV-1 transmission.
Masso M. Masso M. MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review. - DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe?
Soilleux EJ. Soilleux EJ. Clin Sci (Lond). 2003 Apr;104(4):437-46. Clin Sci (Lond). 2003. PMID: 12653690 Review.
Cited by
- Analysis of urinary oligosaccharides in lysosomal storage disorders by capillary high-performance anion-exchange chromatography-mass spectrometry.
Bruggink C, Poorthuis BJ, Deelder AM, Wuhrer M. Bruggink C, et al. Anal Bioanal Chem. 2012 Jun;403(6):1671-83. doi: 10.1007/s00216-012-5968-9. Epub 2012 Apr 20. Anal Bioanal Chem. 2012. PMID: 22526647 Free PMC article. - Human Milk Oligosaccharides: The Journey Ahead.
Ray C, Kerketta JA, Rao S, Patel S, Dutt S, Arora K, Pournami F, Bhushan P. Ray C, et al. Int J Pediatr. 2019 Aug 4;2019:2390240. doi: 10.1155/2019/2390240. eCollection 2019. Int J Pediatr. 2019. PMID: 31467568 Free PMC article. Review. - Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN.
Sabatté J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J, Salamone G, Salomón H, Amigorena S, Geffner J. Sabatté J, et al. J Virol. 2007 Dec;81(24):13723-34. doi: 10.1128/JVI.01079-07. Epub 2007 Oct 3. J Virol. 2007. PMID: 17913809 Free PMC article. - Potential of carbohydrate-binding agents as therapeutics against enveloped viruses.
François KO, Balzarini J. François KO, et al. Med Res Rev. 2012 Mar;32(2):349-87. doi: 10.1002/med.20216. Epub 2010 Jun 23. Med Res Rev. 2012. PMID: 20577974 Free PMC article. Review. - Evolution of DC-SIGN use revealed by fitness studies of R5 HIV-1 variants emerging during AIDS progression.
Borggren M, Repits J, Kuylenstierna C, Sterjovski J, Churchill MJ, Purcell DF, Karlsson A, Albert J, Gorry PR, Jansson M. Borggren M, et al. Retrovirology. 2008 Mar 27;5:28. doi: 10.1186/1742-4690-5-28. Retrovirology. 2008. PMID: 18371209 Free PMC article.
References
- Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. - PubMed
- Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. - PubMed
- Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials