Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors - PubMed (original) (raw)
. 2005 Nov;23(11):1435-9.
doi: 10.1038/nbt1153. Epub 2005 Oct 9.
Affiliations
- PMID: 16244658
- PMCID: PMC2581721
- DOI: 10.1038/nbt1153
Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors
Yi Lai et al. Nat Biotechnol. 2005 Nov.
Abstract
Although adeno-associated virus (AAV)-mediated gene therapy has been hindered by the small viral packaging capacity of the vector, trans-splicing AAV vectors are able to package twice the size of the vector genome. Unfortunately, the efficiency of current trans-splicing vectors is very low. Here we show that rational design of the gene splitting site has a profound influence on trans-splicing vector-mediated gene expression. Using mRNA accumulation as a guide, we generated a set of efficient trans-splicing vectors and achieved widespread expression of the 6-kb DeltaH2-R19 mini-dystrophin gene in skeletal muscle of mdx mice, a model for Duchenne muscular dystrophy. The dystrophic phenotype was ameliorated in both adult and aged mice. This demonstrates the use of trans-splicing vectors to efficiently express a large therapeutic structural protein. This strategy should be applicable to other large therapeutic genes or large transcription regulatory elements.
Figures
Figure 1
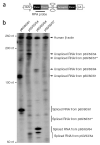
Endogenous splicing signal screening. The RPA was used to evaluate splicing and mRNA accumulation of the selected dystrophin exon/intron/exon junctions in the presence of the dD-ITR structure. (a) Schematic outline of the constructs used in the RPA (not drawn to scale). The endogenous intron splicing sequences were retrieved from genomic DNA by PCR. The dD-ITR was engineered between the 5′ and the 3′ splicing signals inside the intron. The location of antisense RPA probes is marked. (b) A representative photomicrograph from six independent RPA experiments. The endogenous human β-actin RNA (the control for RPA and loading) was detected by an independent probe added at the same time. *, the expected location of the RNA transcripts from p56/56/57.
Figure 2
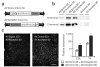
ΔH2 mini-dystrophin expression from two independent sets of _trans_-splicing AAV vectors. The EDL and the TA muscles of 2-month-old mdx mice were co-infected with 1 × 1010 vector genomes (vg) (for the EDL muscle) and 2 × 1010 vg (for the TA muscle) particles of each pair of _trans_-splicing viruses. Single vector infection (donor or acceptor alone) was also included in the cohort study (5 × 109 vg particles for the EDL muscle and 1 × 1010 vg particles for the TA muscle). Mini-dystrophin expression was evaluated 1 month later. (a) Schematic diagram of ΔH2 mini-dystrophin _trans_-splicing vectors (not drawn to scale). The donor virus carries the cytomegalovirus (CMV) promoter, the 5′ portion of the ΔH2 minigene and the endogenous splicing donor signal (from intron 60 and 63, respectively). The acceptor virus carries the endogenous splicing acceptor signal from the same intron (60 and 63, respectively), the remaining minigene and the polyA sequence. The viruses are accordingly named after the respective intron. (b) Western blot analysis of mini-dystrophin expression in muscle extracts with monoclonal N-terminal (specific for human dystrophin) and C-terminal antibodies, respectively. Each lane is from one infected TA muscle. Different samples were used for the N-terminal and C-terminal immunoblots. (c) Quantification of ΔH2 minigene expression by immunofluorescence staining with a human dystrophin–specific antibody. Left panels are the representative photomicrographs from the TA muscles infected with the indicated set of _trans_-splicing vectors. Scale bar, 200 μm. Right panel represents mini-dystrophin-positive myofibers per muscle section. n = 3–7 samples for each group. *, the difference between two sets of _trans_-splicing vectors was statistically significant.
Figure 3
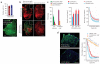
Widespread expression of mini-dystrophin in AV.Donor.60 and AV.Acceptor.60 co-infected mdx muscle attenuates dystrophic pathology. (a) Mini-dystrophin expression in adult mdx TA muscle. 2 × 1010 vg particles of each virus were co-delivered to the TA muscles of 2-month-old male mdx mice. Expression was determined 3 months later by immunostaining. Bar graph represents the transduction efficiency quantified according to immunostaining with monoclonal antibodies. n = 6 for N-terminal antibody and n = 5 for C-terminal antibody. A representative montage composite photomicrograph from an entire TA muscle is shown in the bottom panel. The dystrophin-positive myofibers were revealed by a polyclonal N-terminal antibody. Number in parenthesis indicates the percentage of dystrophin-positive myofibers in the section. Scale bar, 500 μm. (b) Mini-dystrophin expression in adult EDL muscle prevents sarcolemma leakage. Equal amounts (5 × 109 vg particles) of each virus were co-injected into the left EDL muscles of 2-month-old male mdx mice. 5 × 109 vg particles of AV.Acceptor.60 was injected to the contralateral EDL muscles. Mini-dystrophin expression and sarcolemma integrity were examined 3 months later. Photomicrographs are from the representative EDL muscles. Sarcolemma leakage in injured myofibers is revealed by the uptake of immunoglobulin from circulation (anti-mouse lgG staining). Dystrophin expression is detected either by a polyclonal anti-N-terminal antibody (for both mini-dystrophin and revertant dystrophin) or a monoclonal anti-N-terminal antibody (only detects human dystrophin–derived mini-dystrophin). The overlay image of anti-mouse lgG staining (red) and polyclonal antibody immunostaining (green) illustrates the protection of the sarcolemma by mini-dystrophin. Monoclonal antibody immunostaining also reveals membrane leakage in injured myofibers (intense cytosolic staining, different from peripheral membrane staining of dystrophin). Inserts are high power photomicrographs of boxed areas in the corresponding panels. Arrow, immunoglobulin infiltration is prevented in transduced myofibers whereas surrounding cells were not protected. *, revertant myofibers. Scale bar, 400 μm for low power photomicrographs and 50 μm for high power inserts. (c) Quantitative evaluation of muscle pathology and muscle force in the EDL muscles described in panel b. Left panel, quantification of transduction efficiency (n = 15 for co-infection, n = 7 for acceptor only), central nucleation (n = 5 for co-infection, n = 7 for acceptor only), and lgG infiltration (n = 5 for co-infection, n = 4 for acceptor only); middle panel, specific muscle force (n = 7 pairs); right panel, resistance to eccentric contraction-induced injury (n = 7 pairs). *, the difference between co-infected muscle and single vector infected muscle was statistically significant. (d) Efficient mini-dystrophin expression in aged mdx EDL muscle moderately improved muscle function. The left EDL muscles of 1-year-old mdx mice were co-infected with 1.5 × 1010 vg particles of AV.Donor.60 and AV.Acceptor.60 (7.5 × 109 vg particles each). The right EDL muscles were mock-infected with HEPES-buffered saline. Transgene expression, muscle pathology and muscle physiology were examined 6 months later. Left panel, representative photomicrographs of the EDL muscles either co-infected with both AV.Donor.60 and AV.Acceptor.60, or mock infected with HEPES saline. Immunostaining was performed with monoclonal anti-N-terminal antibody. Scale bar, 400 μm. Right panel, resistance to eccentric contraction-induced injury (n = 3 for each group). *, the difference between co-infected muscle and saline-injected muscle was statistically significant according to paired t test. Cross, the results in BL10 muscle were significantly different from that in mdx muscle (both AAV infected and mock treated) according to one-way ANOVA and Bonferroni post hoc test. Pound, the result in BL10 was only significantly better than that of saline-injected mdx muscle according to one-way ANOVA and Bonferroni post hoc test.
Similar articles
- Triple trans-splicing adeno-associated virus vectors capable of transferring the coding sequence for full-length dystrophin protein into dystrophic mice.
Koo T, Popplewell L, Athanasopoulos T, Dickson G. Koo T, et al. Hum Gene Ther. 2014 Feb;25(2):98-108. doi: 10.1089/hum.2013.164. Epub 2013 Dec 19. Hum Gene Ther. 2014. PMID: 24191945 - Trans-splicing adeno-associated viral vector-mediated gene therapy is limited by the accumulation of spliced mRNA but not by dual vector coinfection efficiency.
Xu Z, Yue Y, Lai Y, Ye C, Qiu J, Pintel DJ, Duan D. Xu Z, et al. Hum Gene Ther. 2004 Sep;15(9):896-905. doi: 10.1089/hum.2004.15.896. Hum Gene Ther. 2004. PMID: 15353044 Free PMC article. - Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6.
Odom GL, Gregorevic P, Allen JM, Chamberlain JS. Odom GL, et al. Mol Ther. 2011 Jan;19(1):36-45. doi: 10.1038/mt.2010.205. Epub 2010 Sep 21. Mol Ther. 2011. PMID: 20859263 Free PMC article. - [Gene therapy for muscular dystrophy].
Takeda S. Takeda S. No To Hattatsu. 2004 Mar;36(2):117-23. No To Hattatsu. 2004. PMID: 15031985 Review. Japanese. - Recombinant micro-genes and dystrophin viral vectors.
Dickson G, Roberts ML, Wells DJ, Fabb SA. Dickson G, et al. Neuromuscul Disord. 2002 Oct;12 Suppl 1:S40-4. doi: 10.1016/s0960-8966(02)00080-9. Neuromuscul Disord. 2002. PMID: 12206793 Review.
Cited by
- The sustained expression of Cas9 targeting toxic RNAs reverses disease phenotypes in mouse models of myotonic dystrophy type 1.
Batra R, Nelles DA, Roth DM, Krach F, Nutter CA, Tadokoro T, Thomas JD, Sznajder ŁJ, Blue SM, Gutierrez HL, Liu P, Aigner S, Platoshyn O, Miyanohara A, Marsala M, Swanson MS, Yeo GW. Batra R, et al. Nat Biomed Eng. 2021 Feb;5(2):157-168. doi: 10.1038/s41551-020-00607-7. Epub 2020 Sep 14. Nat Biomed Eng. 2021. PMID: 32929188 Free PMC article. - Engineered DNA plasmid reduces immunity to dystrophin while improving muscle force in a model of gene therapy of Duchenne dystrophy.
Ho PP, Lahey LJ, Mourkioti F, Kraft PE, Filareto A, Brandt M, Magnusson KEG, Finn EE, Chamberlain JS, Robinson WH, Blau HM, Steinman L. Ho PP, et al. Proc Natl Acad Sci U S A. 2018 Sep 25;115(39):E9182-E9191. doi: 10.1073/pnas.1808648115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181272 Free PMC article. - AAV Engineering for Improving Tropism to the Central Nervous System.
Ghauri MS, Ou L. Ghauri MS, et al. Biology (Basel). 2023 Jan 26;12(2):186. doi: 10.3390/biology12020186. Biology (Basel). 2023. PMID: 36829465 Free PMC article. Review. - Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer.
Kwon I, Schaffer DV. Kwon I, et al. Pharm Res. 2008 Mar;25(3):489-99. doi: 10.1007/s11095-007-9431-0. Epub 2007 Sep 1. Pharm Res. 2008. PMID: 17763830 Free PMC article. Review. - Gene Therapy for Heart Failure: New Perspectives.
Gabisonia K, Recchia FA. Gabisonia K, et al. Curr Heart Fail Rep. 2018 Dec;15(6):340-349. doi: 10.1007/s11897-018-0410-z. Curr Heart Fail Rep. 2018. PMID: 30238397 Free PMC article. Review.
References
- Duan D, Yue Y, Engelhardt JF. In: Lung Biology in Health and Disease, Gene Therapy in Lung Disease. Albelda SM, editor. Marcel Dekker Inc.; New York, NY: 2002. pp. 51–92.
- Kay MA, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. - PubMed
- Carter BJ. Adeno-associated virus vectors in clinical trials. Hum. Gene Ther. 2005;16:541–550. - PubMed
- Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996;7:2101–2112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources